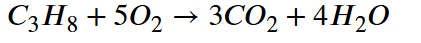give an example of a chemical change
rust, vinegar and baking soda etc
Give an example of a physical change
cutting, melting, splitting, etc
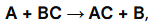
Single Replacement
does forming chemical bonds store or release energy?
forming bonds stores energy,
give an example of an exothermic reaction
explosion, burning wood, etc,
give an example of how cooking is just chemical changes
browning anything on the stove
make a physical change
folding clothes.
break a pencil
rip a paper
Combination/Synthesis
Draw the particles in the 3 phases of matter
solid, liquid, and gas
give 3 signs that a chemical change happened
released bubbles
changed color
precipitate
gave off heat light, etc
cutting paper into strips
lighting paper on fire
putting paper in the freezer
lighting on fire = chemical
Decomposition
Define "chemistry"
the study of matter and how it changes
Endothermic
explain why salt dissolving in water is NOT a chemical change
not making new chemicals, can just evap water and separate them
can be observed about a substance without creating a new substance
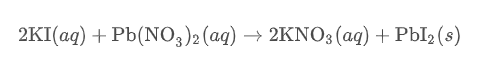
Double Replacement
how many atoms are in the chemical formula: (also what is it?)
C6H12O6
24 atoms total, its glucose
what is the law of conservation of mass
matter cannot be chemically created or destroyed. no matter what chemical reaction happens the matter always needs to end up somewhere.
write a chemical formula showing the process of rusting
iron + oxygen -> rust (iron oxide)
explain how sublimation is a physical change
Not making any new chemicals. If you get the CO2 gas super cold again it will form back into a solid block
Write out the variable versions of all 4 types of chemical reactions. (use A, B, C, D)
A + B -> AB
AB -> A + B
AB + C -> AC + B
AB + CD -> AC + BD
what 3 particles make up an atom?
protons neutrons and electrons
Balance this reaction: