Which type of bond is found in sodium bromide?
What is Ionic Bond?
A bond formed by the transfer of electrons from one atom to another.
What is What is Ionic Bond
Identify the number of atoms in the compound NH4Cl
What is 6?
Why CCl4 nonpolar based on this picture.
What is the chlorines negative charge is evenly spread leading to no polarity?
List 3 characteristics of Ionically Bonded Molecules.
What is :
High Melting point
Hard, brittle solid at room temperature
Poor conductor of heat and electricity as a solid
Good conductor of electricity as a liquid or in an aqueous solution.
Which of the above formulas is polar covalent?
What is H2O and NH3?
As two Chlorine atoms combine to form a molecule, energy is
What is released?
Based on the Properties of Selected Elements chemistry reference table, which atom has the strongest attraction for the electrons in a chemical bond?
What are the Fluorine ?
Highly mobile electrons in the valence shell of metals allows them to be ________, _________ and ________.
List 2 of the 3.
What is a malleable, ductile , and conductive?
Anions have ________ electrons and thus have a _______ charge.
What is gained and negative?
Which substance is correctly paired with its type of bonding?
Br2- polar covalent
NaBr- nonpolar covalent
3. NH3- polar covalent
What is 3?
Draw the electron-dot (Lewis) structure for the NH3 molecule below.
What is 
The bonds between hydrogen and oxygen in a water molecule are classified as_______ ___________.
What is a polar covalent?
Explain why NH3 has stronger intermolecular forces of attraction than Cl2
What is it has polarity and hydrogen bonds (very strong)?
Using the electronegativity on your reference table the bond between which two atoms is most polar
I and F
I and Cl
What is I and F?
What is the correct electron dot diagram for the compound magnesium fluoride
What is
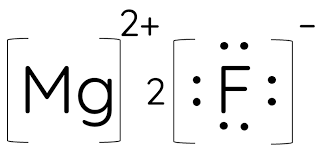
Which element reacts with Oxygen to form ionic bonds.
Nitrogen
Chlorine
Calcium
Hydrogen
What is Calcium?