The atomic number refers to
What holds atoms together in a molecule?
Chemical bond
An ionic bond is between a
metal and nonmetal
A covalent bond is between
Two nonmetals
Turn the name into a formula: dihydrogen monoxide
H2O
How many protons does Phosphorus have?

15
How many atoms of hydrogen in the formula?

2
What happens to the electrons in an ionic bond
Transfer (metal to nonmetal)
What happens to the electrons in a covalent bond
Shared
Name the compound: Fe2O3
Iron Oxide
How many valence electrons does fluorine have?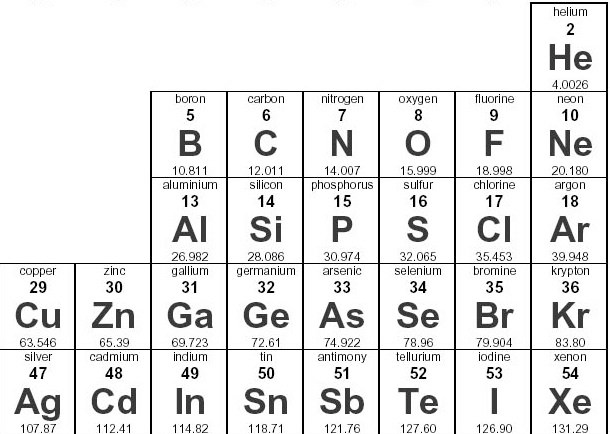
7
How many ELEMENTS are in the formula?

2 (Hydrogen and oxygen)
Name 2 properties of ionic compounds
High melting point, conductive, high boiling point, dissolves in water, crystalline structure
Name 2 properties of covalent compounds
Low melting point, low boiling point, does not conduct, does not dissolve
Name the compound: N2H4
Dinitrogen tetrahydride
What is the oxidation number for oxygen?
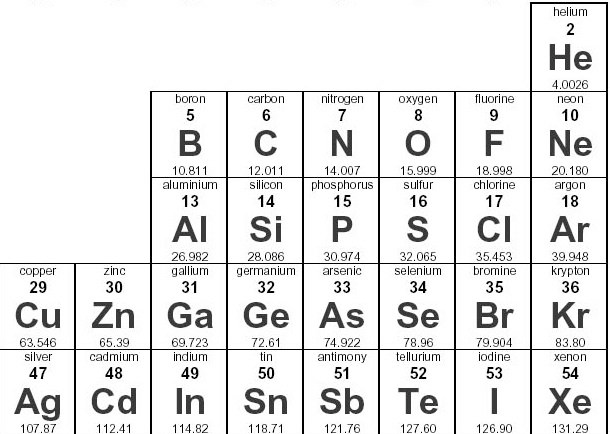
-2
How many ATOMS in the formula?

3 (2 hydrogens + 1 oxygen)
What is the chemical formula for the bond between magnesium and fluorine?
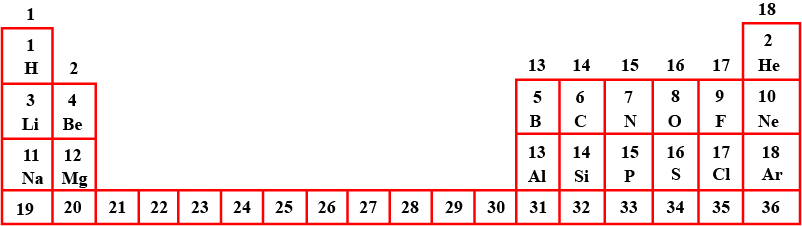
MgF2
How many of EACH atom is contained in the molecule dihydrogen pentoxide?
2 hydrogen
5 oxygen
H2O5
Turn the name into a formula: Magnesium sulfide
MgS
Name one element that has the same oxidation number as Nitrogen
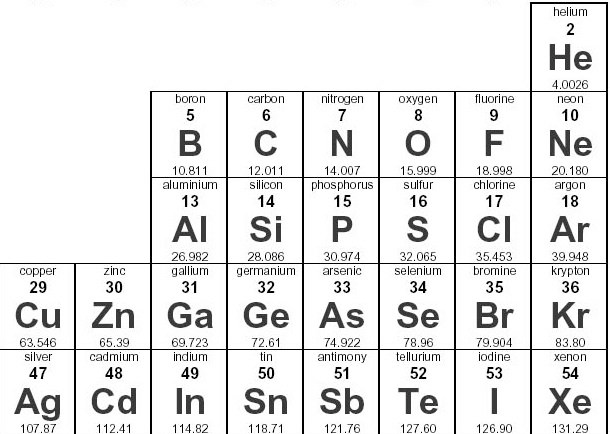
P (phosphorus), As (arsenic), Sb (antimony)
How many total atoms in the formula?
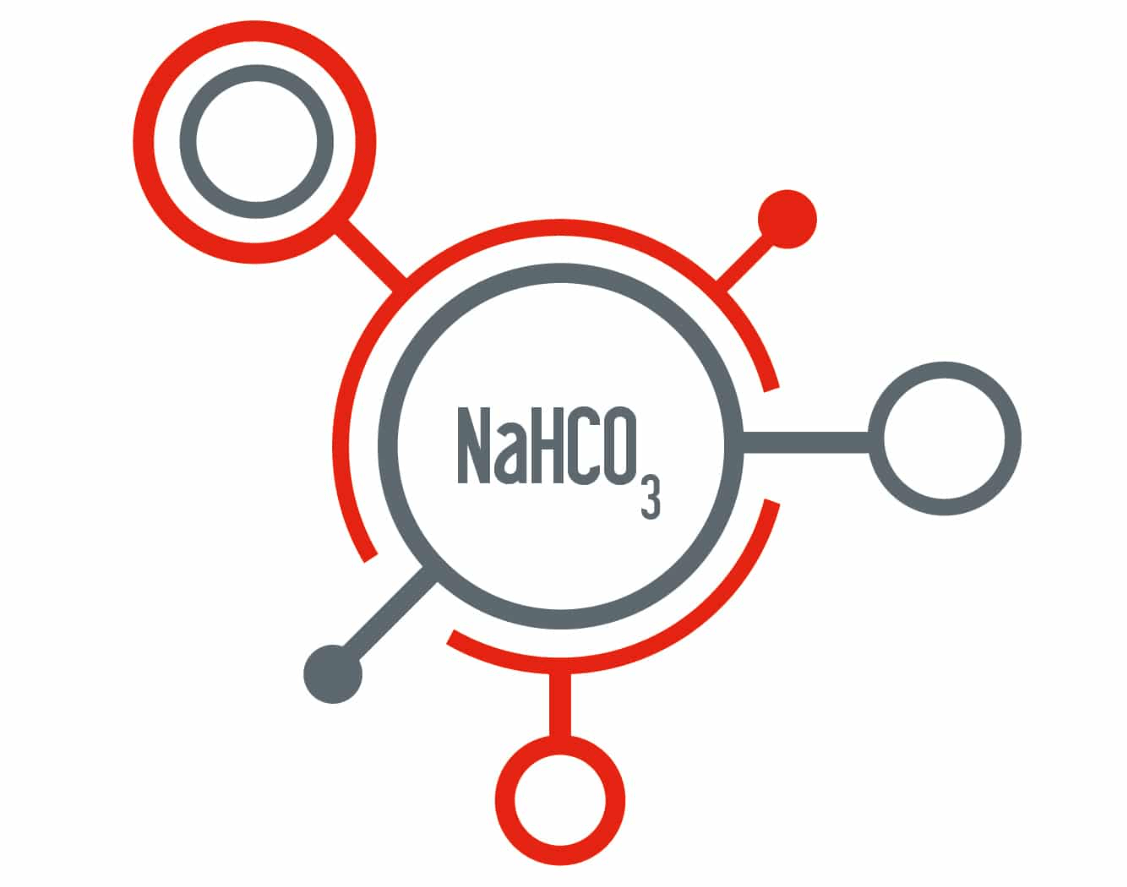
6
What is the chemical formula for the bond between aluminum and oxygen?
Al2O3
How many of EACH atom is contained in the molecule carbon tetrahydride?
1 Carbon, 4 Hydrogen
Turn the name into a formula: aluminum chloride
AlCl3