Why does chemical bonding occur?
to achieve stability
Ionic bonds form between what two types of elements?
Covalent bonds form between what two types of elements?
two nonmetals
Metallic bonds form between what two types of elements?
two metals
Ionic or Covalent?
MgO
Ionic
How many valence electrons does an atom typically need to achieve stability?
eight (8)
What happens to the valence electrons in ionic bonds?
transferred - metal to nonmetal
What happens to the valence electrons in covalent bonds?
shared
How do we describe the electrons within a metallic bond?
"sea of electrons"
Ionic or Covalent?
CO2
Covalent
What group on the periodic table does not participate in chemical bonding due to having a full valence shell?
noble gases
Write the formula and name for a bond between Magnesium & Bromine
MgBr2
Magnesium Bromide
CO2
What property describes a metal's ability to be hammered into thin sheets?
Malleability
Rank the following IMF's from strongest to weakest:
London Disperion, Hydrogen Bonding, Dipole-Dipole
Hydrogen Bonding, Dipole-Dipole, London Dispersion
How many valence electrons (lewis dots) would calcium have?
2
Write the formula and name for a bond between Iron with a 3+ charge and Oxygen
Fe2O3
Iron (III) Oxide
Write the name for CF4
Carbon Tetrafloride
What property describes a metal's ability to be drawn into wires?
Ductility
What is the name for the acid H2CO3?
Carbonic Acid
What is the charge (oxidation number) for phosphorus?
-3
Write the formula and name for a bond between Barium & Phosphate
Ba3(PO4)2
Barium Phosphate
Draw the lewis dot structure for H20 and write its molecular geometry
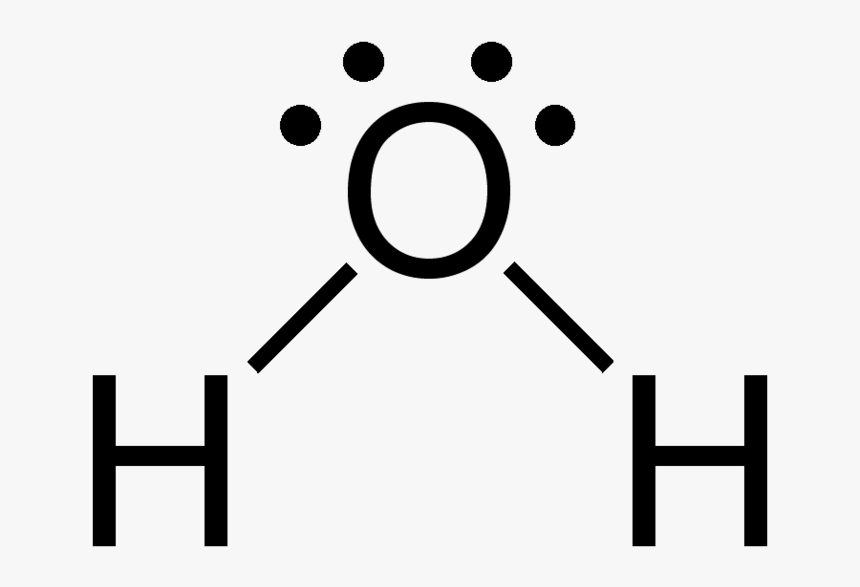
Bent
Metals are good conductors of ______ and _______.
Heat & Electricity
What poison killed Anabelle Smith?
Thalium Sulfate