Valence Electrons
Lewis-Dot Structures
Covalent Bonds
Ionic Bonds
100
How many valence electrons does Hydrogen have?

1
100
Draw a Lewis-Dot Structure for Chlorine.

100
Covalent Bonds occur between what type(s) of elements?
Nonmetals.
100
Ionic Bonds occur between what type(s) of elements?
Metals and Nonmetals*
(You'll later learn that this is more appropriately written as Cations and Anions)
200
How many valence electrons does Sodium have?

1
200
Draw a Lewis-Dot Structure for Sulfur (Sulphur).

200
How many covalent bonds can Oxygen make?
2
200
How many Sodium atoms would it take to balance a Nitrogen atom's charge?
3
300
How many valence electrons does Carbon have?

4
300
Write the number of paired electrons and single electrons Beryllium has in its valence shell.
0 pairs, 2 single electrons.
300
Which of the following will not covalently bond with Chlorine?
Oxygen, Sodium, Carbon, Nitrogen, Sulfur.
Sodium, because it is a metal and metals cannot make covalent bonds.
300
What is Calcium's oxidation number?
+2
400
How many valence electrons does Iodine have?

7
400
Write the number of paired electrons and single electrons Nitrogen has in its valence shell.
1 pair of electrons, 3 single electrons.
400
Draw the bond structure for propane gas, C3H8
400
What is the chemical formula (like H2O) for the bonding of Calcium and Iodine?
CaI2
500
How many valence electrons does Gold have?

2
500
Draw a Lewis Dot structure for Francium.
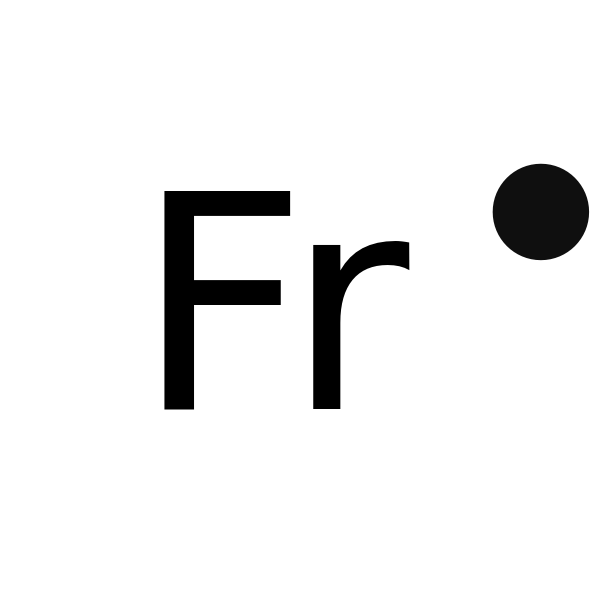
500
Ethanol, C2H6O, is an alcohol found in moonshine and gasoline.
Draw the bond structure for ethanol. It's based around the two carbons connected by a single covalent bond.
500
Write a chemical formula (like H2O) for the bonding of Aluminum and Oxygen.
Al2O3