Why do elements form bonds?
To have full valence shells , to be stable
A positively charged ion is called a/an _____.
cation
Have a high conductivity.
True or False. You must explain your choice.
False, covalent bonds are between non-metals and non-metals.
Does CO2 contain ionic or covalent bonds?
covalent
Which group does not tend to form bonds?
Noble gases
This bond is when two or more atoms share electrons.
covalent bond
A negatively charged ion is called a/an _____.
anion
True or False: All elements can have up to eight valence electrons.
False. (ex: hydrogen and helium need only 2)
Does HF contain covalent or ionic bonds?
covalent
Which type of atoms tend to give away electrons?
metals
The bond formed when two or more atoms transfer electrons.
ionic bonding
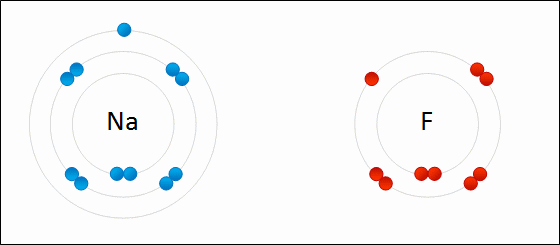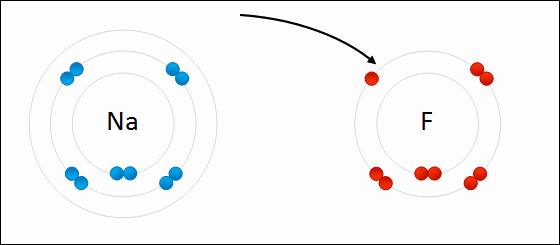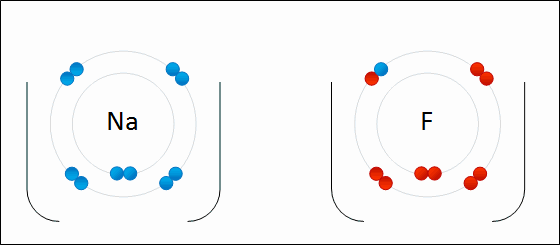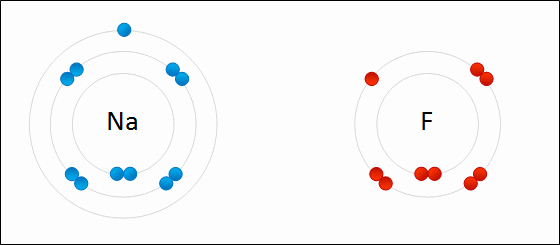
Alkali metals will always ionize to form ions with a ____ charge.
1+
How many bonds will a nitrogen atom have with chlorine atoms?
3
Does Li2O contain ionic or covalent bonds?
ionic
Why don't ionic bonds form between either metal and metal or nonmetal and nonmetal?
Because metals tend to give electrons and nonmetals tend to take electrons.
If an atom loses an electron, then that atom will become ____ charged.
positively charged
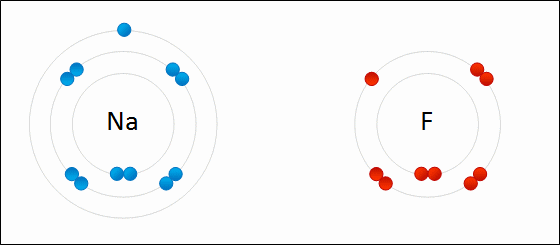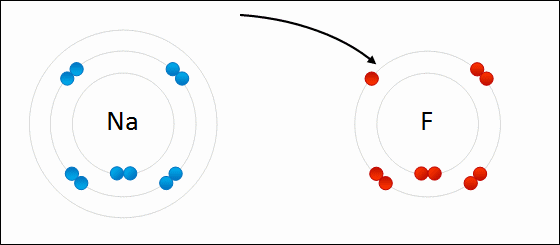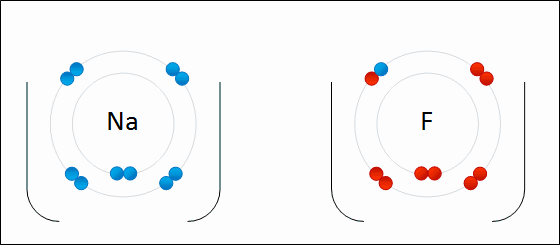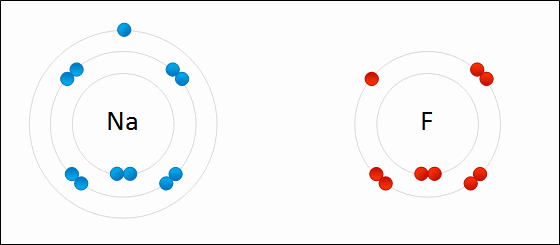
Go up to the board and draw the bonding of table salt
tearcher corrects
Ionic bonds can exist as solids, liquids or gases.
True or False
True
What did your wonderful, intelligent, gracefully aged professor have you repeat over and over about the elements in the groups on the periodic Table
They have similar characteristics
In a hydrocarbon compound, how many bonds will form on each carbon atom?
a) 4 ionic bonds, b) 4 covalent bonds , c) 8 ionic bonds d) 8 covalent bonds
You May pass question to other team, if they answer correctly they get double the points
B) 4 covalent bonds
If an atom gains an electron, then that atom will become ____ charged.
negatively charged

Ionic bonds always have a high melting point and a low boiling point. True or False
False. The boiling point is also high
Have lower melting and boiling points.
True or False
True
In the oxygen molecule (O2), how many electrons are shared?
4 electrons
Which element will form a covalent bond with oxygen
a) sodium B) lithium c) nitrogen d) magnesium
You can pass , if other team answers correctly they get double points
C). Nitrogen
Which electrons are responsible for the reactivities of the elements?
valence electrons
outermost electrons
electrons in outermost shell
Why do the atoms stay together after transferring electrons?
They're oppositely charged, so they attract each other. Electrostatic force.
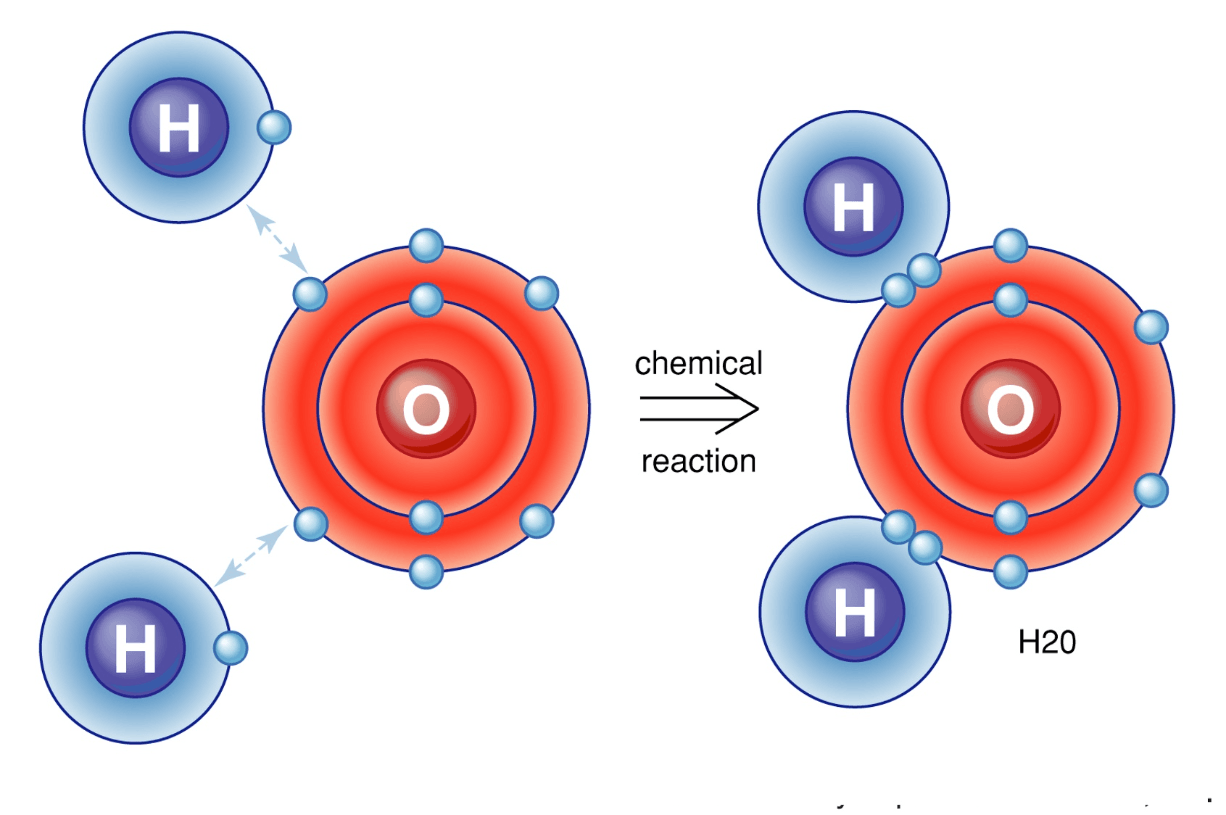
go up to the board and draw a picture of the bonding in H2O. All must be correct
Teacher corrects.
When the C2H4 molecule is drawn, how many electrons will be shared between the two carbon atoms?
4 electrons
Draw the formation of a covalent bond between carbon and 2 oxygen, CO2.
teacher corrects