No!
What three subatomic particles make up atoms?
Protons, electrons, neutrons
What are three main types of chemical bonds?
Covalent, ionic, and hydrogen bonds.
Quadruple Jeopardy!
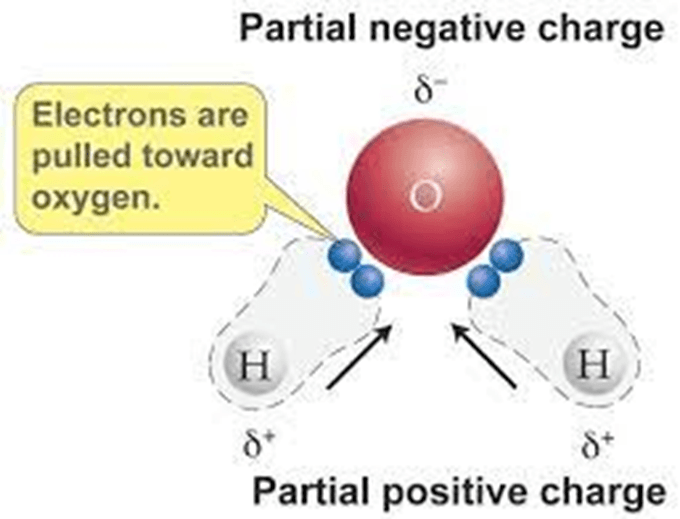
Water(H2O) is a _______________ ________________ because the hydrogen is slightly ______________ and the oxygen is slightly ________________. Water molecules form ____________________ bonds with neighboring molecules
Polar Molecule, positive, negative, hydrogen
Triple Jeopardy!
The higher the amount of _______________ in a ______________, the greater the ___________________.
Solute, Solvent, Concentration
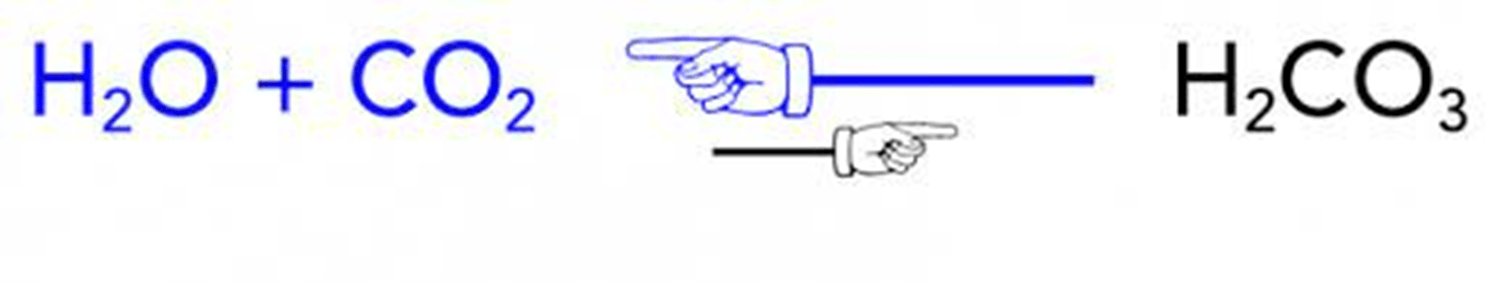
_____________ ________________change substances into different substances by breaking chemical bonds and forming new chemical bonds, rearranging atoms in the process.

Chemical Reactions
What type of bond is represented below, and how do you know:
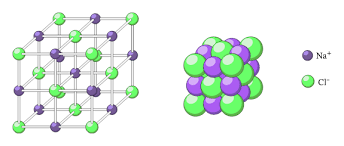
Ionic = Metal + Nonmetal
What are the charges on each subatomic particle:
Proton
Neutron
Electron
Proton (+)
Neutron (0)
Electron (-)
An atom that has lost electrons is called a(n)_____________ and has a ______________charge.
cation, positive
What are the for major properties of water (H2O)?
Cohesion
Adhesion
High Specific Heat
Solvency
An ____________ is a substance that donates _____________ ________, while a__________ is a substance that removes them.
Acid, hydrogen ions (H+), base
In the following reaction, what is the reactant(s) and what is the product(s).
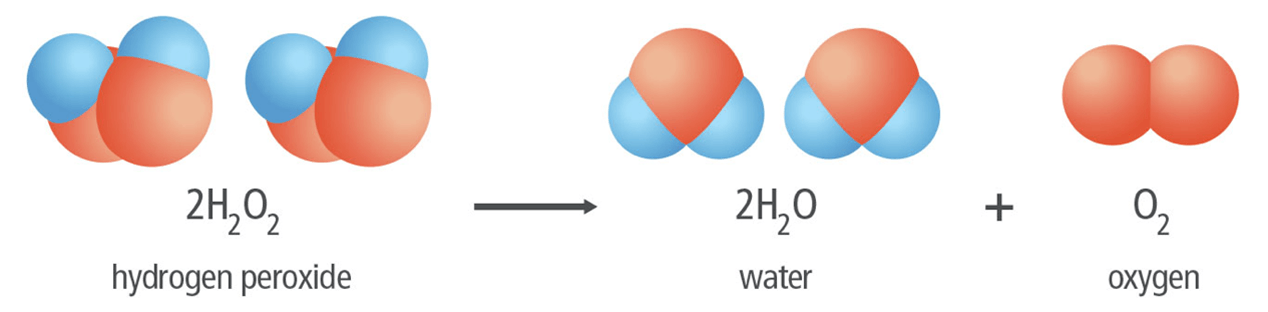
Hydrogen peroxide -> Water and Oxygen
What type of bond is represented below and how do you know?
Covalent Compound = nonmetal + nonmetal
Label the following diagram:
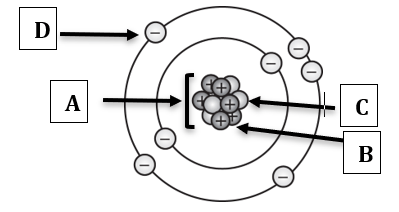
A) Nucleus
B) Proton
C) Neutron
D) Electron
An atom that has gained electrons is called a(n)___________ and has a _____________ charge.
anion, negative
Water is known as a "________________ ______________", because it can dissolve most substances in nature. 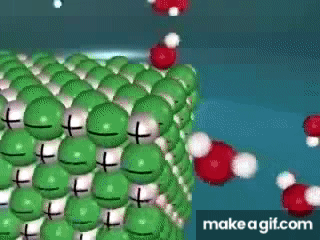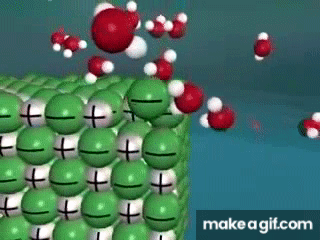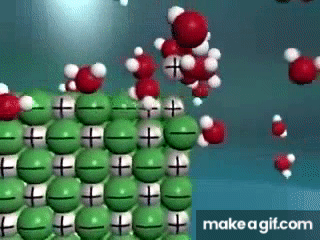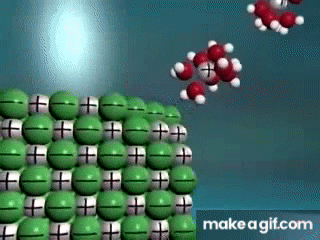
"Universal Solvent"
Complete the following:
A) pH range of 7 = ______
B) pH range of >7 = ______
C) pH range of <7 = ______
A) Neutral
B) Basic
C) Acidic
Double Jeopardy!
According to the ________________ ___ _______________, the product(s) of this reaction should have _______carbon, ________oxygen, ________Sodium, and _________ hydrogens.
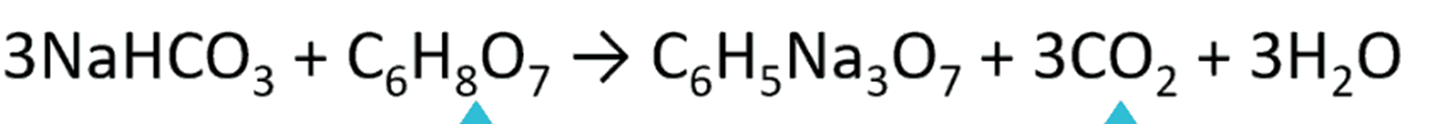
conservation of matter, 9, 16, 3, 11
What type of bond is represented below:
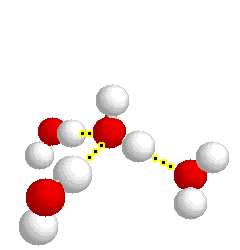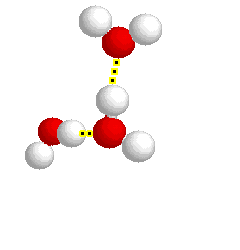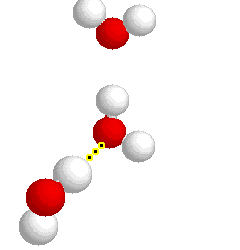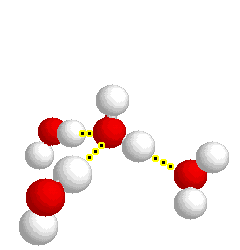
hydrogen bond
Double Jeopardy!
The ________________ ________________ is equal to the number of _______________ in the nucleus; and determines the ________________ of an element.
Atomic number; protons, identity
Double Joepardy
Usually metals lose electrons and form ________________.
Nonmetals tend to gain electrons and form______________.
Cations, anions
Image A is an example of ________________. Image B is an example of _______________.


____________bind to H+ ions and prevent blood from becoming too Acidic.
Buffers
Double Joepardy!
_________________ _________________ proceed in one direction from reactants to products. _________________ _______________ may proceed in both directions between reactants and products until the reaction reaches __________________
Irreversible reactions, reversible reactions, equilibrium
Double Jeopardy! In the following diagram, Maltose is a ____________ that binds to the ___________ __________ of the enzyme maltase. It is then broken down into two molecules of glucose. Excess pH and heat can ______________ enzymes
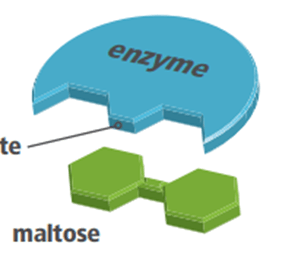
substrate, active site, denature
What element has 84 protons in its nucleus
Polonium
This type of bond forms between metals and nonmetals
Ionic bonds
Double Jeopardy!
High Specific Heat
Double Jeopardy!
Of the following substances, which dissolve which?
A) Water
B) Mechanic grease (nonpolar)
C) Gasoline (nonpolar
D) Sugar/salt
A dissolves D
C dissolves B
"Like dissolves like"
Double Joepardy!
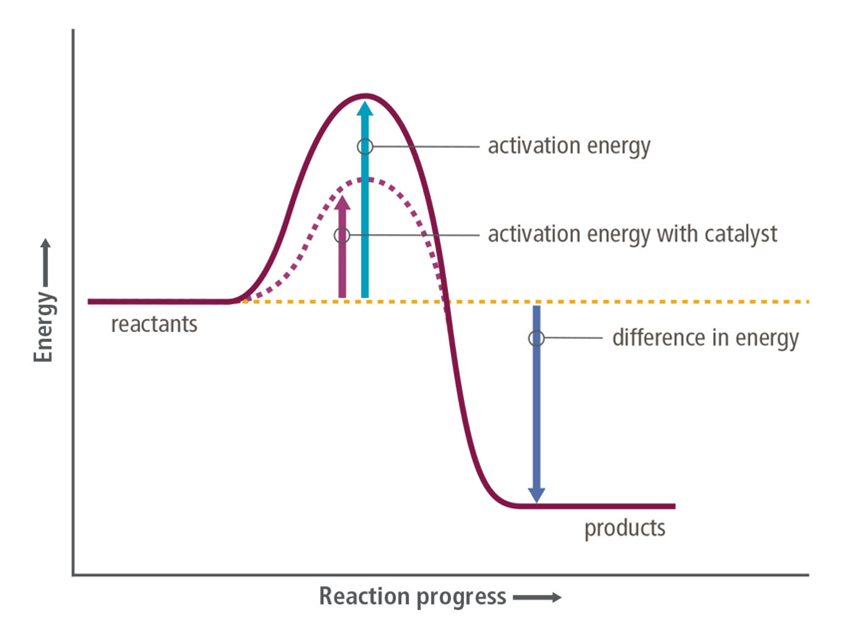
In biological systems, a(n) _____________ is a type of _____________ which increases the rate of a reaction without being consumed. It helps speed up reactions by ___________ the ____________ _______________.
Enzyme, catalyst, lowering, activation energy
The following demonstration illustrates an ______________ reaction, because heat is lost from the environment.

endothermic
What is the atomic number of palladium
46
Double Jeopardy!
___________ bonds form between a nonmetal and a nonmetal. They form so that each atom has _________outer electrons for stability. The chemical combination of two different elements forms __________________
covalent bonds, eight (8), compounds
Double Joepardy
A __________________is made up of two parts. The _______________ is the substance that dissolves, and the _______________ is the substance that's being dissolved.
Solution, solvent, solute
Double Joepardy!
Phenolphthalein is an indicator that turns bright pink when a solution becomes basic. In the following demonstration, what is happening to hydrogen ions?

Hydrogen Ions are being removed from the solution.
Double Joepardy!
___________ absorb energy to break chemical bonds. ____________ release energy when new bonds are formed. The amount of energy required to break a bond is called the ____________ ___________. It may be related to the _____________ ______________ which is the amount of energy required to start a chemical reaction
Reactants, products, bond energy, activation energy
Double Jeopardy! When a chemical reaction releases more energy than it absorbs, it is_________________. When a chemical reaction absorbs more energy than it releases, it is________________. The following is an example of an _______________ reaction.

Exothermic, Endothermic, Exothermic