What is the name of the bond formed by the sharing of electrons?
Covalent bond
What does VSEPR stand for?
Valence Shell Electron Pair Repulsion
What must the total charge of an ionic compound be?
Zero (neutral)
What is the type of reaction where one element replaces another in a compound?
Single replacement reaction
What is the unit of measurement for the amount of a substance, often used in stoichiometry?
Mole
Which type of bond typically forms between a metal and a nonmetal?
Ionic bond
What shape does a molecule with 3 electron domains and no lone pairs have?
Trigonal planar
What is created when atoms in a covalent compounds unevenly shar eelctrons?
Dipole
In which type of reaction does one substance break down into two or more products?
Decomposition reaction
How do you convert grams to moles?
Divide by the molar mass
What rule states that atoms tend to gain, lose, or share electrons to achieve a full set of 8 valence electrons?
Octet Rule
What bond angle is associated with a linear molecular geometry?
180°
Why do ionic compounds conduct electricity when dissolved in water but not as solids?
In water, the ions are free to move, allowing the flow of electricity, whereas in solids, ions are locked in place.
What reaction involves the transfer of electrons between species?
Redox (oxidation-reduction) reaction
What is the oxidative state of carbon in the oxalate ion?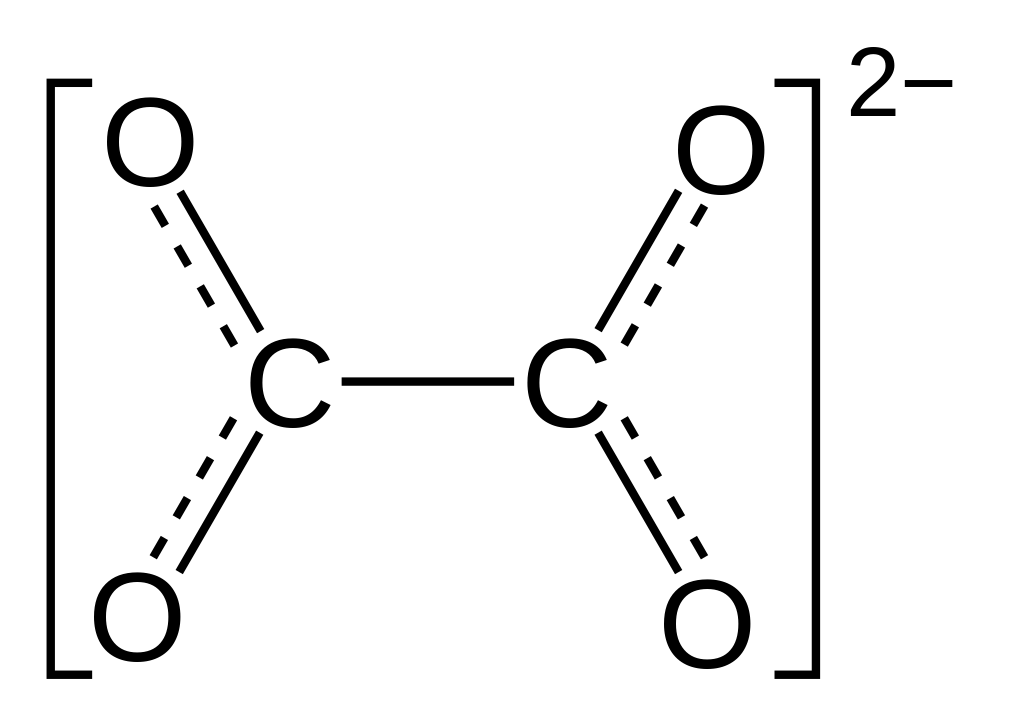
+3
What type of bond shares electrons according to the Electron Sea Theory?
Metallic bond
What is the molecular geometry of a molecule with 4 electron domains and 2 lone pairs?
Bent (4)
What is the term for mixture of metals that still function like pure metals?
Alloy
What type of reaction mixes two solutions to form a solid?
Precipitation reaction
Balance the following reaction.
KClO3 -> KClO4 + KCl
4KClO3 → 3KClO4 + KCl
Name one exception to the octet rule.
Molecules with an odd number of electrons, molecules with fewer than eight electrons, or molecules with more than eight electrons (expanded octet)
What kind of hybridization does the central atom have in methane?
sp3
What term is used to describe the energy required to separate one mole of ions in an ionic compound?
Lattice energy
What type of reaction involves the exchange of hydrogen ions and usually produces water?
Acid-Base Reaction
What is the limiting reactant in a reaction?
The reactant that is completely consumed and limits the amount of product formed