Write the formula for calcium fluoride
CaF2
What is the mass of 1.5 moles of CO2?
66 g
Which type of chemical reaction is represented by the equation?
F2(g) + 2KCl(aq)-->2KF(aq) + Cl2(g)
Single Replacement
The coefficients in a balanced chemical equation represent
A) the mass ratios of the substances in the reaction
B) the mole ratios of the substances in the reaction
C) the total number of electrons in the reaction
D) the total number of elements in the reaction
B) the mole ratios of the substances in the reaction
How many moles of carbon dioxide are produced when 4 moles of C4H10 react completely?
2C4H10 + 13O2 8CO2 + 10H2O + energy
16 moles
Write the formula for sodium sulfate
Na2SO4
One mole of O2 has approximately the same mass as one mole of
A) CH4
B) S
C) LiH
D) Cl2
B) S
Which type of reaction does this equation represent?
2NaCl-->2Na + Cl2
decomposition
What is the mole ratio of oxygen molecules to carbon dioxide molecules in the following equation?
C2H5OH()+3O2(g) 2CO2(g)+3H2O() + energy
3:2
What is the number of moles of C that must completely react to produce 2.0 moles of C2H6?
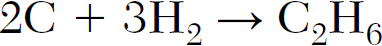
4 moles
Write the formula for aluminum iodide
AlI3
What is the number of moles of CO2 in a 220.-gram sample of CO2 (gram-formula mass = 44 g/mol)?
5.0 mol
Which equation represents a single replacement reaction?
A) 2Al(s) + 3Cl2(g) 2AlCl3(s)
B) 2Al(s) + 6HCl(aq) 2AlCl3(aq) + 3H2(g)
C) 2AlCl3(s) 2Al(s) + 3Cl2(g)
D) AlCl3(aq) + 3KOH(aq) Al(OH)3(s) + 3KCl(aq)
B) 2Al(s) + 6HCl(aq) 2AlCl3(aq) + 3H2(g)
What do the coefficients in a balanced chemical equation represent?
moles
What is the number of moles of H2O(g) formed when 2.0 moles of NH3(g) react completely?
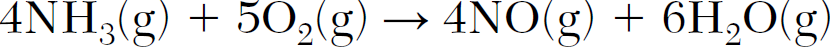
3 moles
What is the chemical formula for zinc carbonate?
ZnCO3
What is the number of moles in a 78.8-gram sample of MgCO3 (gram-formula mass = 84.3 g/mol)?
0.935 mol
Classify the following reaction:
NaCl(aq) + AgNO3(aq) NaNO3(aq) + AgCl(s)
Double Replacement
Which chemical equation is correctly balanced?
A) H2(g) + O2(g) → H2O(g)
B) N2(g) + H2(g) → NH3(g)
C) 2NaCl(s) → Na(s) + Cl2(g)
D) 2KCl(s) → 2K(s) + Cl2(g)
D) 2KCl(s) → 2K(s) + Cl2(g)
What is the mole ratio of NaOH to Al(OH)3 in the following equation:
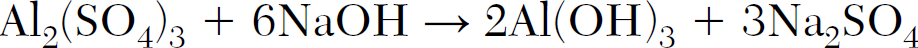
6:2 or 3:1
Write the formula for aluminum hydroxide
Al(OH)3
What is the mass in grams of 2.0 moles of NO2?
92
Which terms identify types of chemical reactions?
A) decomposition and sublimation
B) decomposition and synthesis
C) deposition and sublimation
D) deposition and synthesis
B) decomposition and synthesis
Balance the following equation:
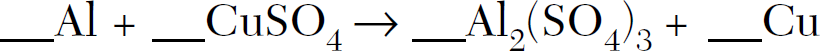
2Al + 3CuSO4-->1Al2(SO4)3 + 3Cu
What is the total number of moles of O2(g) required for the complete combustion of 1.5 moles of C3H8(g)?
C3H8(g) +5O2(g) 3CO2(g) + 4H2O(g)
7.5 moles