Non-metal, halogen, period 2
Fluorine, F
Amount (how much of something is present)
Physical property
Alkali metals
Element
Group 2A, metal, period 4
Ca, Calcium
Odor (smell of substance)
Physical property
Halogens
These are classified as ______
CO, H2O, NH3
Compounds
Transition metal, neighbors with copper
Zn, Zinc
Combustion (burning of a compound with oxygen to produce carbon dioxide and water)
Chemical property

Noble gases
These are examples of _______
F, Br, Cs
Elements
Group 1A, alkali metal, period 6
Cs, Cesium
Solubility (the ability to dissolve in solution, usually water)
Physical property
Transition metals
A substance made by joining atoms together
Compound
Liquid, metal, transition metal
Hg, Mercury
Toxicity (the quality of being toxic or poisonous)
Chemical property
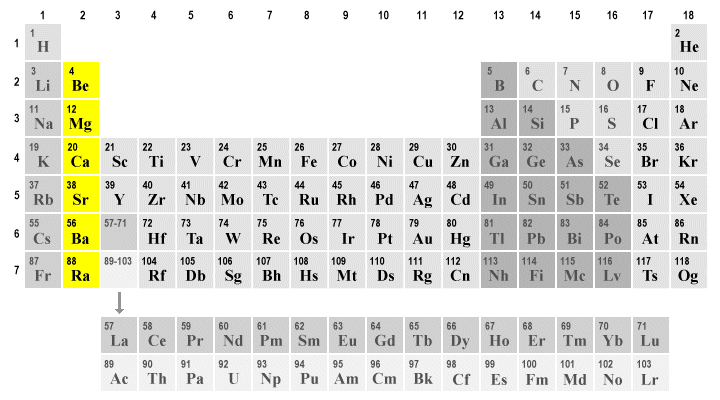
Alkaline earth metals
The group of elements that only exist in nature as compounds are called ______
(HINT: an example is O2)
Diatomic elements
