Chemical or Physical Change
Removing thermal energy from water so it freezes
Physical
Two or more reactants combine to form a product
Synthesis
__KBr + __ Mg -> __MgBr + __ K
KBr + Mg -> MgBr + K
___________ are biological catalysts
Enzymes
How many grams are in 1 kg
1000 grams
Find the prepositional phrase:
During the winter, Bob went skiing
During the night
Physical or Chemical Change
Copper turning Green when exposed to oxygen
Chemical
A reactant breaks apart into two or more products
decomposition
__NaCl + __ F2 -> __ NaF + __ Cl2
2 NaCl + F2 -> 2 NaF + Cl2
A reaction that takes energy from the surrounding environment
endothermic
Liquid
Who painted the Mona Lisa?
Leonardo Da Vinci
Formation of bubbles indicates what is produced?
Gas
AB + C -> AC + B
Single Replacement
__H2O + ___O2 -> ___H2O2
2H2O + 1 O2 -> 2 H2O2
What do we call the energy required to start a chemical reaction
Activation Energy
Name for the group or family of elements that most easily donate electrons
The Alkali Metals
Group 1 (for half points)
What Gas was used in this blimp disaster
Hydrogen
Physical Or Chemical
Two solutions are mixed in a vial, the vial gets cold
Chemical
AB+ CD -> AD + CB
Double Replacement
__N2 + __ H2 -> __ NH3
N2 + 3H2 -> 2NH3
Endothermic or Exothermic
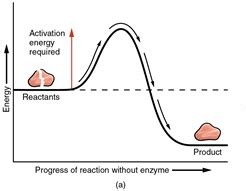
Exothermic
Name this ionic molecule
CaCl2
Calcium Chloride
A majority of the US Founding Father's all owned ________
slaves
A name for a solid that is produced during a chemical reaction
precipitate
CxHz + O2 -> H2O + CO2
Combustion
__C8H18 + ___O2 -> ___CO2 + ___H2O
2C8H18 +25O2 -> 16CO2 + 18H2O
Breaking bonds _______ energy while Forming new bonds ___________ energy
absorbs, releases
Name for group or family of elements that accept electrons the most readily
The Halogens
Group 7 (For half points)
Add the linear expressions
(-4x+2) + (-2x +2)
(-6x+4)