Is water boiling a physical or chemical change?
Physical
A + B -> AB
Synthesis (Addition) reaction
How many hydrogen atoms are in H2O?
2
Balance this chemical equation
H2 + O2 -> H2O
2H2 + O2 -> 2H2O
What side of a chemical equation are the products on?
right
Is bread being toasted a physical or chemical change?
Chemical
2H2O -> 2H2 + O2
Decomposition reaction
How many carbon atoms are in CO2?
1
Balance this chemical equation
CH4 + O2 -> CO2 + H2O
What are the names of the little numbers on elements in chemical equations?
subscripts
Chemical
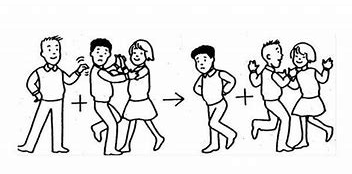
Single replacement reaction
How many nitrogen atoms are in 3N2?
6
Balance this chemical equation
C2H5OH + O2 -> CO2 + H2O
C2H5OH + 3O2 -> 2CO2 + 3H2O
True or false
Exothermic: Energy is being absorbed
Endothermic: Energy is being released
False!
Can a physical change be undone?
Yes
C2H4 + 3O2 -> 2CO2 + 2H2O
Combustion Reaction
On the right side of this unbalanced equation, how many oxygen atoms are there?
CaCl2 + 2NaHCO3 -> 2NaCl + CaCO3 + CO2 + H2O
6
Balance this chemical equation
HCl + NaOH → NaCl + H2O
It is already balanced
How many types of chemical reaction are there?
Five
List 3 indicators that a chemical change is taking place.
Heat, light, production of a gas, color change, and the formation of a precipitate
BaCl2 + Na2SO4 → BaSO4 + 2NaCl
Double replacement reaction
On the reactants side of the equation, how many hydrogen atoms are there?
2C6H14 + 19O2 -> 12CO2 + 14H2O
28
Balance this chemical equation
C3H8 + O2 → CO2 + H2O
C3H8 + 5O2 -> 3CO2 + 4H2O
What is the law of conservation of mass?
The number of atoms on the reactants (left) side of a chemical equation will be the same on the products (right) side of the equation.