What do we call the substances that react in a chemical reaction, written on the left side of a chemical equation?
Reactants
What do we call a process by which two or more chemical substances interact and are chemically changed, producing different chemical substances?
Chemical reaction
In a physical change, a new substance (is/is not) created.
Choose is or is not.
Is not
What is a molecule?
2 or more atoms
What is the law of conservation of mass?
MATTER CANNOT BE CREATED OR DESTROYED.
Magnesium sulfate + sodium bicarbonate → ________________ sulfate + magnesium bicarbonate
sodium
What's one observable sign of a chemical reaction?
There's lot's of answers, e.g.:
a temperature change
the formation of a precipitate
an explosion
light is released
a color change
a gas is released
Cutting a piece of paper is an example of a ________ change.
Physical
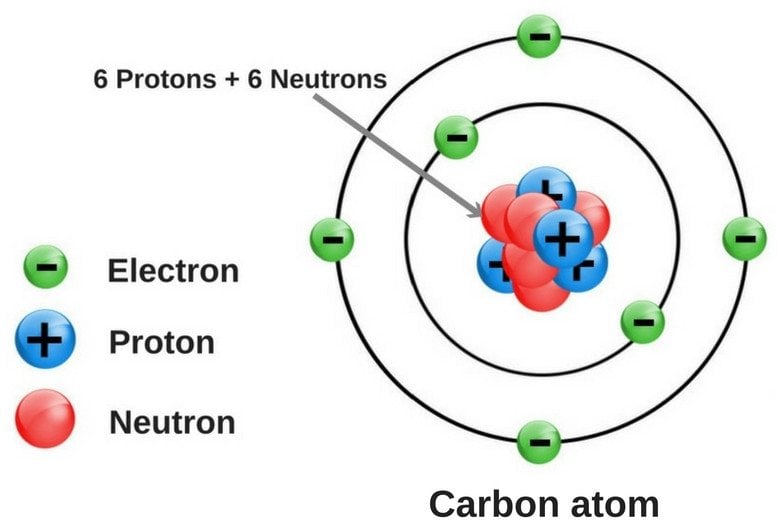
What are the 3 parts, or subatomic particles, that make up atoms called? And what are their charges (positive, negative, or neutral)?
Protons, (+)
Electrons, (-)
Neutrons, (neutral)
What is mass?
Mass is how much something weighs.
Write a word equation that models how mercury metal and oxygen combine to form mercuric acid:
___________ + __________ --> _________________
mercury metal + oxygen --> mercuric oxide
This substance is called a precipitate.
It is a sign of a chemical reaction.
Bacteria forming inside a container of spoiled milk is an example of a ________ change.
Chemical

If red represents hydrogen (H) atoms, and blue represents carbon (C) atoms, write the chemical equation for this molecule.
CH4
In a chemical reaction, the mass, or weights, of the products and the reactants are:
1) Different
2) The same
3) It depends on the reaction
2) THE SAME.
Balance the following chemical equation.
__H2 + __O2 --> __H2O
2H2 + O2 --> 2H2O
What happens to the atoms in a chemical reaction?
They rearrange to form new molecules.
Alfonza has some delicious ice cream that he got on a hot summer day. To his dismay, his ice cream begins to melt in the heat from the sun. Did Alfonza's ice cream go through a physical or a chemical change?
Physical
Compounds are molecules that contain more than one element. Which of the following molecules are compounds?
H2
C4H5
NaCl
Fe2
Br3
H2O
C4H5
NaCl
H2O
If we have 2 grams of oxygen gas that combines with 1 gram of hydrogen gas in a chemical reaction and produces water, how many grams of water did we produce?
3 grams of water
Balance the following chemical equation:
__P + __O2 --> __P2O5
4P + 5O2 --> 2P2O5
In a chemical reaction, when atoms rearrange, can matter be created or destroyed?
No. Matter cannot be created or destroyed. This is called the law of conservation of mass.
Which of the following are chemical properties--meaning, in order to observe them, we have to change the chemical composition of a substance?
flammability
shape
reactivity to water
volume
boiling point
luster
pH
flammability, reactivity to water, and pH
Draw an accurate model of this atom:
Make up your own chemical equation using real elements and BALANCE it to reflect the law of conservation of mass.
Answers may vary! As long as there are equal amounts of atoms on each side, you're correct!