This type of change alters the chemical bonds in a substance.
What is a chemical bond?
This is the name of the substances on the left side of a chemical equation
What are reactants?
This is absorbed to break bonds in the reactants
What is energy?
Zn + S → ZnS is this kind of reaction.
What is an Addition Reaction?
Balance MgF2 +Li2CO3 → MgCO3 +LiF
MgF2 +Li2CO3 → MgCO3 + 2 LiF
This type of change does not result in a new substance.
What is a physical change?
This law states that the number of atoms in the reactants must equal the number of atoms in the products
What is the Law of Conservation of Mass?
When a chemical reaction releases more energy than it absorbed
What is an exothermic reaction?
Cl2 + 2 KBr → 2 KCl + Br2 is this kind of reaction
What is a single-displacement reaction?
Balance H2 + O2 → H2O
2H2 + O2 → 2H2O
This type of change occurs when cooking a pancake
What is a chemical change?
These parts of a chemical reaction may include atoms, compounds, or energy
What are reactants and products?
This type of reaction will feel cool or cold to touch
What is an endothermic reaction
This type of reaction will change the nucleus of an atom
What is a nuclear reaction?
Balance Pb(OH)2 + HCl → H2O + PbCl2
Pb(OH)2 + 2 HCl → 2 H2O + PbCl2
This type of change occurs when a liquid evaporates.
What is a physical change?
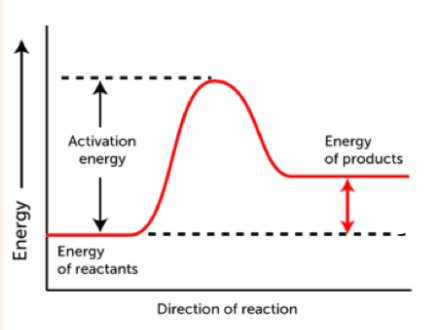 This graph describes this kind of reaction
This graph describes this kind of reaction
What is an endothermic reaction?
C4H12 + 7 O2 → 6 H2O + 4 CO2 is this kind of reaction
What is combustion?
Balance C4H12 + O2 → H2O + CO2
C4H12 + 7 O2 → 6 H2O + 4 CO2
Physical changes and chemical changes have this natural law in common
What is the law of conservation of mass?
This is the amount of energy required to start a chemical reaction
What is activation energy?
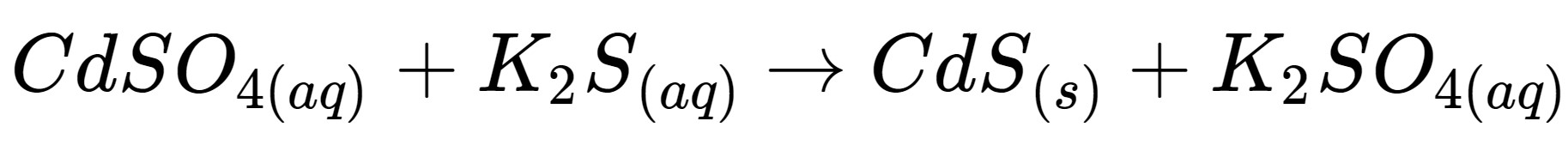 This is this kind of reaction
This is this kind of reaction
What is a precipitation reaction?
Balance Na3PO4 + CaCl2 → NaCl + Ca3(PO4)2
2 Na3PO4 + 3 CaCl2 → 6 NaCl + Ca3(PO4)2
C6H12O6 + 6O2 → 6CO2 + 6H2O + energy
is this kind of reaction
What is an exothermic reaction?
This type of addition reaction results in several molecules being combined to form one long chain
What is polymerization?