What are the 3 components of collision theory?
1. molecules must collide to react
2. molecules must collide with sufficient energy
3. molecules must collide at the correct orientation
You dissolve a salt in water and the beaker becomes cold. This is an example of an ______ reaction.
endothermic
___ NH4F + ___ AlCl3 → ___ NH4Cl + ___ AlF3
3 NH4F + AlCl3 → 3 NH4Cl + AlF3
1. Give the reaction type
2. Predict the product(s)
3. Balance the equation
C5H12 + O2 -->
Combustion
C5H12 + 8 O2 --> 5 CO2 + 6 H2O
Write the chemical formulas for one soluble ionic compound and one insoluble ionic compound.
Answers may vary.
Soluble examples: NaCl, Mg(NO3)2, NH4OH
Insoluble examples: AgCl, CaCO3, PbSO4
Name 4 ways to increase the rate of a reaction.
1. increase temperature
2. increase concentration
3. increase surface area
4. add a catalyst
What is the difference between an endothermic and an exothermic reaction?
Endothermic reactions absorb energy, exothermic reactions release energy.
__ PbSO4 → __ PbSO3 + __ O2
2 PbSO4 → 2 PbSO3 + O2
1. Give the reaction type
2. Predict the product(s)
3. Balance the equation
Li(s) + O2(g) -->
Synthesis
4 Li + O2 --> 2 Li2O
What are the spectator ions in the complete ionic equation below?
Na+ + Cl- + Ag+ + NO3- --> Na+ + NO3- + AgCl
Na+ and NO3-
Draw a diagram showing how surface area influences reaction rate.

Give 2 real life examples of endothermic and exothermic reactions (4 total).
Answers may vary.
Endothermic- ice melting, water evaporating
Exothermic- combustion, glow stick, hot pack
___ Ni + ___ H2SO4 → ___ H2 + ___ Ni2(SO4)3
2 Ni + 3 H2SO4 → 3 H2 + Ni2(SO4)3
1. Give the reaction type
2. Predict the product(s)
3. Balance the equation
Ca(ClO3)2 -->
Decomposition
Ca(ClO3)2 --> CaCl2 + 3 O2
Write the complete ionic equation for the following:
3Ba(NO3)2(aq)+ 2Na3P(aq)-->Ba3P2(s)+ 6NaNO3(aq)
3Ba2+ + 6NO3- + 6Na+ + 2P3- -->
Ba3P2 + 6Na+ + 6NO3-
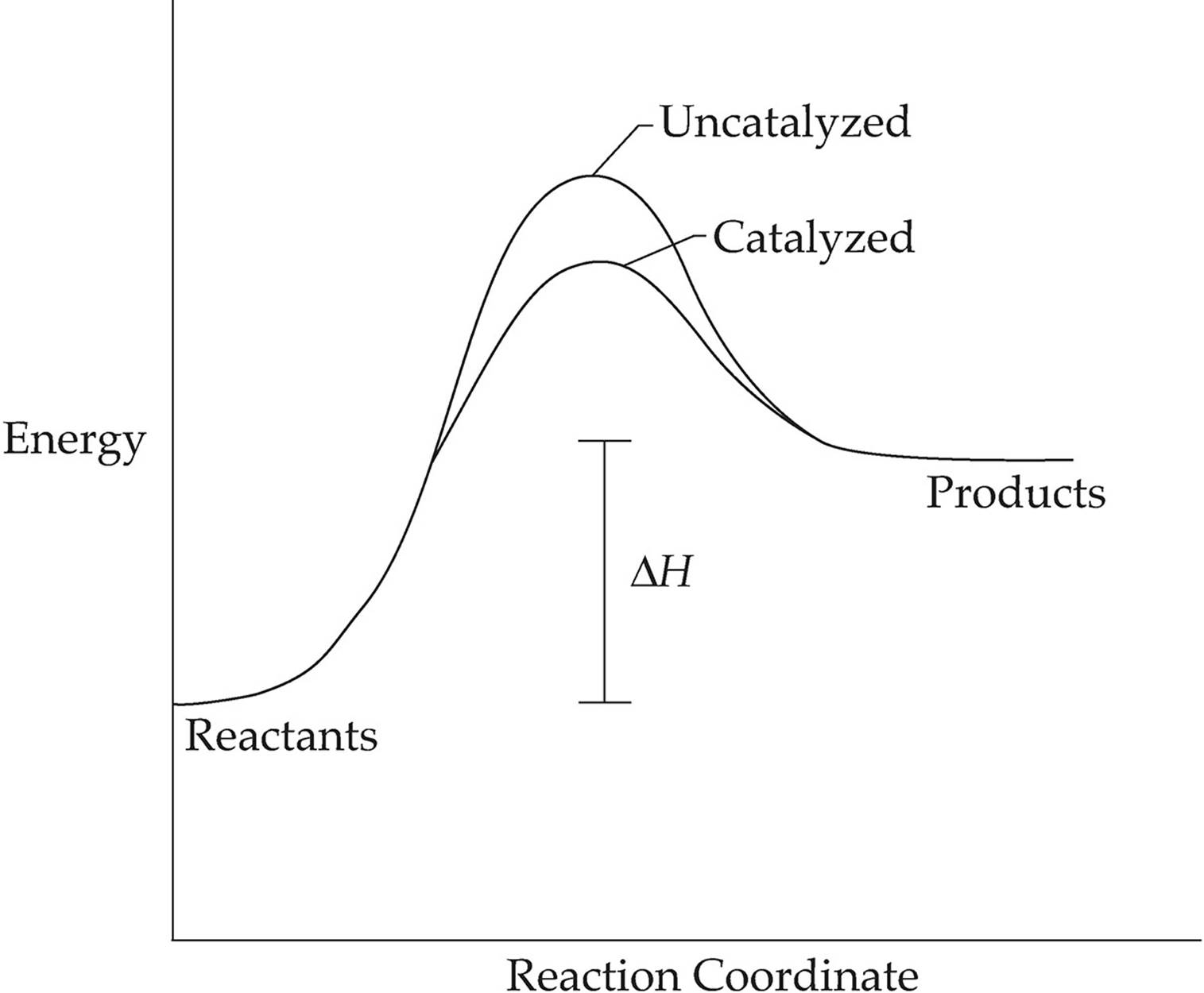
How does a catalyst work to change the rate of a reaction? Draw an energy diagram showing an uncatalyzed and a catalyzed reaction.
Draw the energy diagram for an endothermic reaction. Label the overall energy change, the reactants and products, and the activation energy
_ Na2CO3 + _ H3PO4 → _ Na3PO4 + _ H2O + _ CO2
3 Na2CO3 + 2 H3PO4 → 2 Na3PO4 + 3 H2O + 3 CO2
1. Give the reaction type
2. Predict the product(s)
3. Balance the equation
H2SO4 + Mg(OH)2 -->
Acid-Base
H2SO4 + Mg(OH)2 --> MgSO4 + 2 H2O
Write the molecular, complete ionic, and net ionic equations.
HBr + LiOH -->
HBr(aq) + LiOH(aq) --> LiBr(aq) + H2O(l)
H+ + Br- + Li+ + OH- --> Li+ + Br- + H2O
H+ + OH- --> H2O
Draw an effective collision for the reaction below, representing each atom with a different color circle.
Cl + NOCl --> NO + Cl2
Molecules have to absorb energy to break bonds, and energy is released when bonds are formed. The stronger the bond, the more energy is absorbed or released.
Describe the relative strengths of the bonds broken and bonds formed in an exothermic reaction.
The bonds formed are stronger than the bonds broken.
___ C4H8O + ___ O2 → ___ CO2 + ___ H2O
2 C4H8O + 11 O2 → 8 CO2 + 8 H2O
1. Give the reaction type
2. Predict the product(s)
3. Balance the equation
Al + HNO3 -->
Single Replacement
2 Al + 6 HNO3 --> 3 H2 + 2 Al(NO3)3
Write the molecular, complete ionic, and net ionic equations.
lithium chromate + barium chloride -->
Li2CrO4(aq) + BaCl2(aq) --> 2 LiCl(aq) + BaCrO4(s)
2Li++CrO42-+Ba2++2Cl- --> 2Li++2Cl-+BaCrO4
CrO42- + Ba2+ --> BaCrO4
