What subatomic particle determines the identity of an atom?
Electron
Proton
Neutron
Shells
Proton
What is known as avogadro's number?
6.022x10^23
What type of bond forms between a metal and non metal?
Ionic Bond
What gas do plants release that humans breathe
Oxygen
Which particle has almost no mass?
Protons
Neutrons
Electrons
Hydrogen
Electrons
How many moles are in 3.01x10^23 atoms of carbon?
0.5
Draw the lewis dot structure of H2O
H-O-H
BONUS (500 points)
Mr Davila's least favorite element is what?
Krypton
An atom with 38 Neutrons and 40 protons will make the atom have a mass of what?
78amu
Write the nuclear symbol of an atom with 19 protons, 20 neutrons, and 18 electrons
K+
Convert 25g of H2O to moles
1.39 mol
Write the VESPR formula of 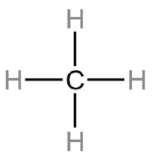
AB4
What is the most abundant element in the universe?
Hydrogen
What is the molar mass of Cl2
70
Which has a greater radius: Na or Na+
Na(neutral atom) because losing electrons reduces the number of shells Na has, making the radius smaller
How many molecules are in 18 grams of water?
6.022x1023
The lewis dot structure of Flourine looks like..
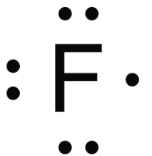
Which scientist dicovered the law of gravity
Newton
In the first energy level how many electrons can be in it?
2
What is the mass number of an atom with 15 protons, 17 neutrons, and 16 electrons?
32
Calculate the number of atoms in 2.5 mol of CO2
4.52x1024
How does C4H10 look like when bonded in a lewis dot structure
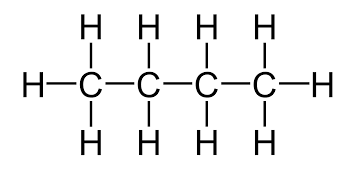
What is the metal that is liquid at room temperature
Mercury
The element in group 14 Period 2 is what?
Carbon