Phase in which molecules that are are far apart as possible and has an undefined shape
What is a "gas"?
This is the table in which heat of Fusion and Heat of vaporization can be found?
What is Table B?
The name of this graph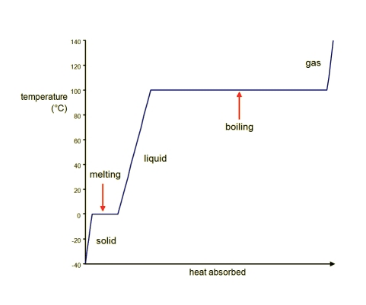
What is a "heating curve"?
The specific heat capacity of water
What is 4.18 J/g°C?
As pressure stays constant and temperature increases, volume will do this.
What is "increase"?
This type of mixture consists of sand and water at room temperature
What is a "heterogenous mixture"?
Given the balanced equation representing a reaction:
![]()
What type of reaction is this?
What is an exothermic reaction?
True or False, average kinetic energy decreases at the flat plateaus on the cooling curve
False, it neither increases nor decreases
This symbol deltaT represents this in the specific heat formula
What is "change in temperature"?
According to the Kinetic Molecular Theory, gases move in random, __________ lines, and the volume is negligible.
What are "straight" lines?
This type of mixture's components can vary.
What is a "heterogenous mixture"?
A 50.0-gram block of copper at 100.0°C is carefully lowered into 100.0 grams of water at 10.0°C in an insulated container. Which statement describes the transfer of heat in this system?
What is "heat transfer from the copper block to the water"
This potential graph describes the following type of reaction.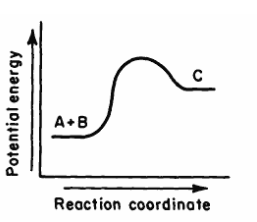
What is an "endothermic reaction"?
Specific type of energy that is related to heat:
a) electrical energy
b) chemical energy
c) mechanical energy
d) thermal energy
What is "thermal energy"?
200.-milliliter sample of CO2(g) is placed in a sealed, rigid cylinder with a movable piston at 296 K and 101.3 kPa. Determine the volume of the sample of CO2(g) if the temperature and pressure are changed to 336 K and 152.0 kPa.
What is "151 mL or 151.5 mL"?
Which process can be used to separate a mixture of two liquids having different boiling points
What is a "distillation"?
Solid zinc has a specific heat capacity of 0.90 J/g•K. How many joules of heat are absorbed to raise the temperature of 32.0 grams of aluminum from 200 K to 350. K?
What is 4320 J?
On cooling curve, this plateau is when a gas becomes a liquid
What is "condensation"?
Mrs. Alkayfee wants to make of muffins in 38g of WATER, how much energy would her oven's WATER need to absorb to raise the temperature from 22°C to 350°C?
What is 52099.52 J?
Determine the volume of the helium gas when the pressure is increased to 1.50 atm and the temperature remains at 25.0°C.
What is 100mL?
I am a phase that can fill any room.
I am the phase that has the highest kinetic energy.
I am the phase that gets spaced out easily.
I am a gas?
Addition of a catalyst can speed up a reaction by providing an alternate reaction pathway that has a
What is "lower activation energy"?
The segment of the graph that exists as a liquid and a solid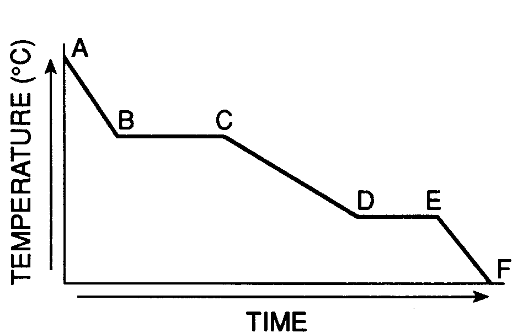
What is "Segment DE"?
The numerical setup can be used to calculate the heat energy required to completely melt 100. grams of H2O(s) at 0°C
What is (100g)(334 J/g)?
A sample of gas occupies a volume of 50.0 milliliters in a cylinder with a movable piston. The pressure of the sample is 0.90 atmosphere and the temperature is 298 K. What is the volume of the sample at STP?
What is 41 mL?
Identify the following phase change: I2(s) --> I2(g).
What is sublimation?
What is the amount of heat required to completely melt a 200.-gram sample of H2O(s) at STP?
What is "66800 J"?
Part of the graph that is changed by the addition of a catalyst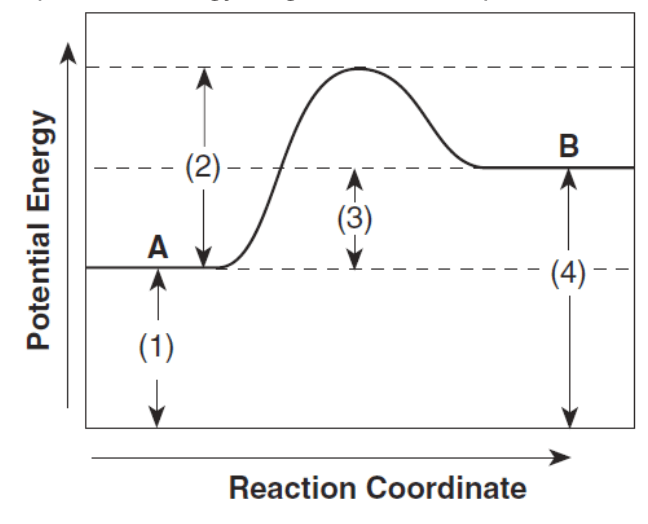
What is "B"?
The Law that explains the relationship between pressure and volume
What is Boyle's law/Indirect relationship