A system loses 1150 J of heat and has 480 J of work done on it. What is the change in enthalpy (delta E)?
-670 J
What is the daughter nucleus if Uranium-238 undergoes Alpha-decay?
(A) Thorium-234
(B) Thorium-236
(C) Neptunium-234
(D) Uranium-234
(A) Thorium-234
What are the two factors that make a solution ideal?
Low concentration and similar size solutes and solvents
How many moles of gas would you have if you had a volume of 38.0 L under a pressure of 1432 mmHg at standard temperature?
1.70 mol
2Mg(s) + O2 --> 2MgO(s)
deltaH = -1204 kJ
Is the reaction endothermic or exothermic?
Calculate the amount of heat transferred when 3.55g Mg(s) reacts at constant pressure.
Exothermic, -87.93 kJ
Give the Van't Hoff factor of MgCl2.
i = 3
What determines how strong an acid is?
How many ions it forms in a solution.
34.0 mL of 6.0 M sulfuric acid solution is spilled on the floor. The acid is neutralized by pouring sodium hydrogen carbonate on the spilled acid. What is the volume, in L, of the carbon dioxide which is released at 25 deg. C and 1 atm?
H2SO4(aq) + 2NaHCO3(s) --> Na2SO4(aq) + 2H2O(l) + 2CO2(g)
9.98 L
What is the difference between crystalline and amorphous solids?
Crystalline solids have repeating patterns while amorphous solids are characterized by their lack of order
What are the (a) mole fraction and (b) molality of ethylene glycol, C2H4(OH)2, in a solution prepared from 2.22 × 103 g of ethylene glycol and 2.00 × 103 g of water (approximately 2 L of glycol and 2 L of water)?
a) 0.244
b) 17.9 mol/kg
What volume of hydrogen at 225 atm and 35.5 °C would be required to react with 1 ton (1.000 × 103 kg) of CCl2F2 in the following equation:
CCl2F2(g)+4H2(g)⟶CH2F2(g)+2HCl(g)
3.72 ×103 L
Use average bond energies to estimate deltaHrxn for the following reaction:
4NH3(g) + 7O2(g) --> 4NO2(g) + 6H2O(g)
N-H = 391 kJ/mol N-O = 201 kJ/mol
O=O - 495kJ/mol O-H = 463 kJ/mol
993 kJ/mol
Name the phase change from a solid directly to a gas.
Sublimation
A solution is made containing 42.8 g. of phenol (C6H5OH) in 358 g of ethanol (CH3CH2OH). What is the mole fraction of ethanol?
0.94
What is the total volume of the CO2(g) and H2O(g) at 600 °C and 0.888 atm produced by the combustion of 1.00 L of C2H6(g) measured at STP?
18.0 L
Determine the standard enthalpy of formation for ethanol:
C2H5OH(l)+3O2(g)⟶2CO2(g)+3H2O(l)
−1366.8 kJ/mol
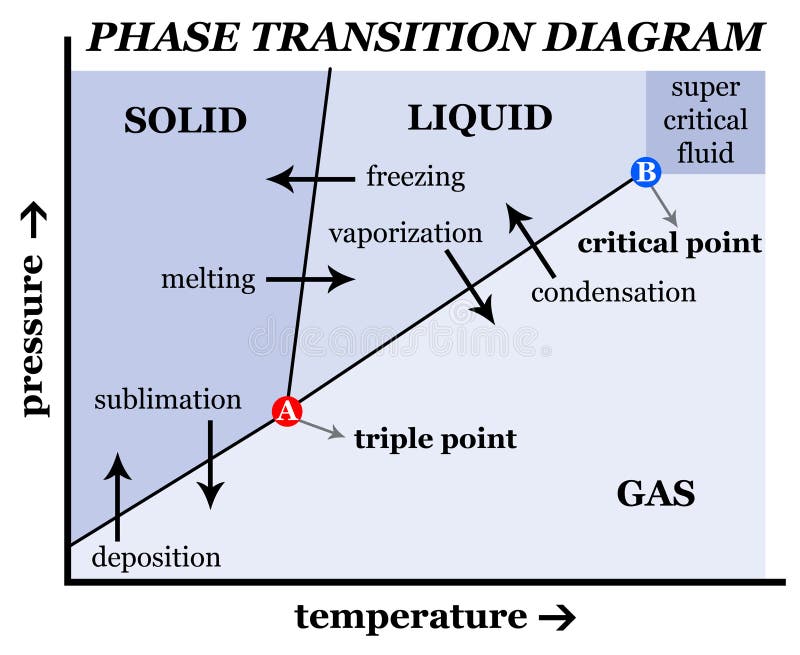
Give the labels for the following blank phase diagram:

Assuming ideal solution behavior, what is the boiling point of a 0.33 m solution of a nonvolatile solute in benzene? (Kb = 2.53 deg. Cm-1) (Boiling point = 80.1 deg. C)
80.9 deg. C