What are the three particles that make up an atom?
Protons, Neutrons, and Electrons
How many elements are there total on the periodic table?
118
Matter is defined as anything that has _____________ and _____________________
Mass and Volume
(Atoms and takes up space)
Where do you find an atom's VALANCE ELECTRONS?
in the outermost energy shell
Why would you need to use the eye wash in lab?
If you get chemicals in your eyes
What two particles of an atom are found in the nucleus and make up that atom’s mass
Protons and Neutrons
columns
What are the metric base units that we use for MASS
grams
What is the 3-number rule for the first 3 energy levels of electrons?
how many electrons can fit in the first 3 shells?
____-____-____
2-8-8
Explain what you should do as a student when there is broken glass in lab
DO NOT TOUCH THE BROKEN GLASS
Tell your teacher immediately
make sure all broken glass goes in the special container
What is the definition of a MOLECULE?
Two or more atoms bonded together
Name three properties of metals (100 pts for each)
Ductile
Malleable
Conductors
Luster
Almost all are solid at room temperature (except mercury)
magnetism
:max_bytes(150000):strip_icc()/meniscus01-58b5b2c03df78cdcd8ab8299.png) What is the volume of the liquid?
What is the volume of the liquid?
24 ml
Fin the number of protons, neutrons, and electrons in a stable atom of iron
P = 26
E = 26
N = 30 (56-26)
What is my son's first name???? (300 pts)
What is my son's middle name? (100 bonus pts)
EVERETT HUDSON ROSENBARKER :)
Explain how you would calculate the number of neutrons in an atom
Take the atomic mass and subtract the number of protons to get neutrons
1) What group/family number are the alkali metals
2) why are alkali metals so reactive?
200 pts each
1) group/family #1
2) because they all have one valance electron
What is the total mass shown on the triple beam balance?
77.5 g
1) Draw the Bohr model for an uncharged atom of Nitrogen
2) State how many valance electrons there are
 5 Ve-
5 Ve-
What is the volume of the rectangular box pictured on the board?
30 cm
How many total atoms of hydrogen are there in the REACTANTS of the chemical equation below:
Photosynthesis:
6(CO2) + 6(H2O) → C6H12O6 + 6(O2)
12
6H20 = h20 6 times
2 x 6 = 12
Write down one METALLOID
(100 bonus pts if you can name two)
Boron, germanium, silicon, antimony, arsenic, tellurium and pollanium
Explain how you would find the volume of an irregularly shaped object by using the displacement method
Fill a container with a starting amount of water, place object into that container (or another container and collect overflow), then measure how much the water rose.
What would happen if you put a noble gas with an alkali metal, AND explain why this would happen
Nothing... noble gasses are non-reactive because their valance electron shell is always FULL.
(full shells = happy atoms :)
The noble gas would prevent the alkali metal from reacting.
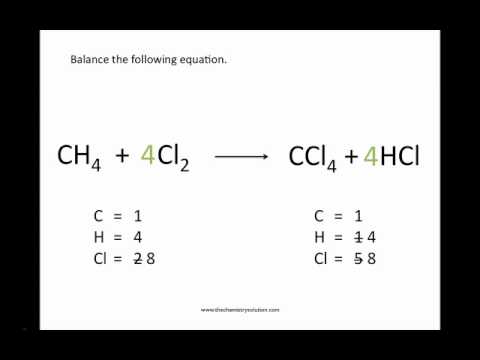
Balance the following chemical equation:
C=carbon, H=hydrogen, Cl=chlorine,
__CH4 + ___Cl2 ---> ___CCl + ___HCl