Which phrase describes the nucleus of any atom?
A) has an overall positive charge
B) has an overall negative charge
C) contains negative electrons
D) contains positive electrons
B) has an overall positive charge
Diamond and graphite are two forms of solid carbon. These two forms of carbon have
A) different crystal structures and different properties
B) different crystal structures and the same properties
C) the same crystal structure and different properties
D) the same crystal structure and the same properties
A) different crystal structures and different properties
A potassium atom has a mass number of 37. What is the number of neutrons in this atom?
A) 15
B) 18
C) 22
D) 37
B) 18
Given the bright-line spectra of four elements and the spectrum of a mixture formed from two of these elements:
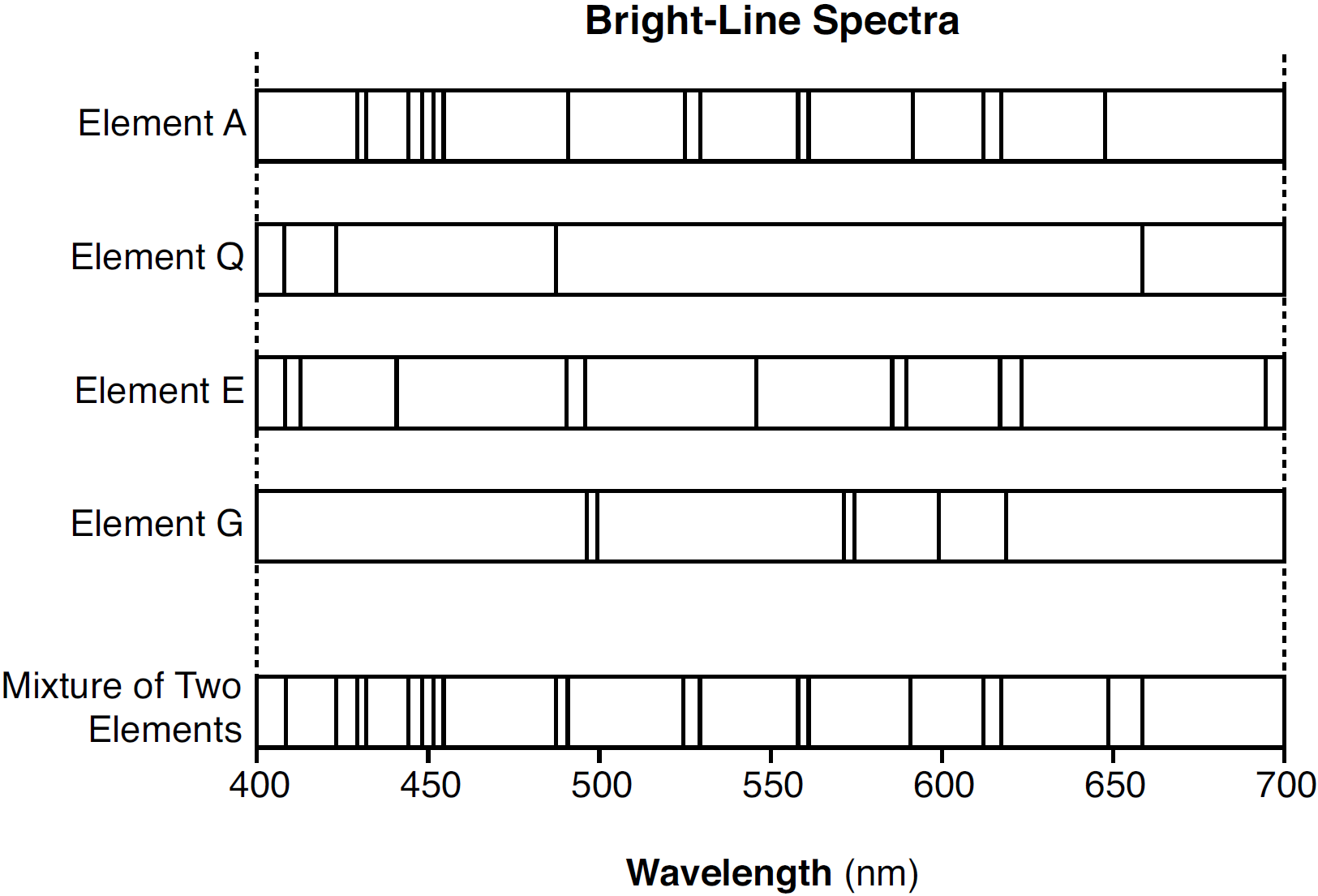
Which two elements are present in this mixture?
A) A and Q
B) A and E
C) G and Q
D) G and E
A) A and Q
A nuclear reaction is represented by the equation below.
![]()
Explain why the equation represents a transmutation.
Hydrogen-3 is converted to helium-3.
Which two particles each have a mass of approximately one atomic mass unit? (Table O)
A) an electron and a proton.
B) an electron and a positron
C) a neutron and a proton
D) a neutron and a positron
C) a neutron and a proton
Which substance can be broken down by a chemical change? (Check Periodic Table/ Table S)
A) cobalt
B) ethane
C) krypton
D) manganese
B) ethane
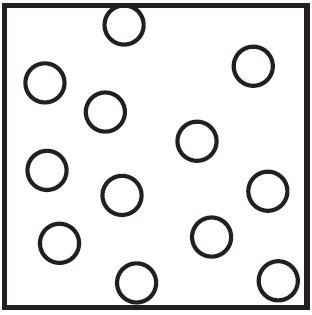
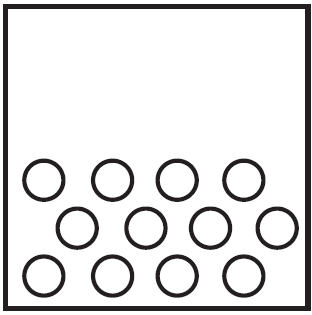
Which particle diagram represents a sample of xenon at STP?
A)
B)
C)
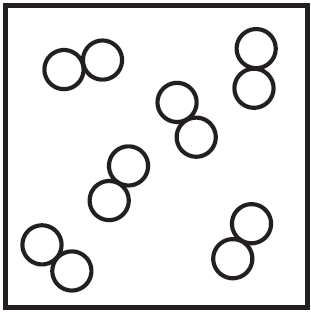
D)
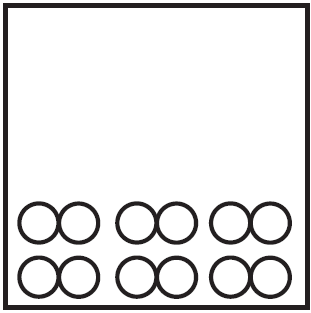
A)

Which electron configuration represents the electrons of an atom in an excited state?
A) 2-7-3
B) 2-8-2
C) 2-8-8-1
D) 2-8-9-2
A) 2-7-3
Which nuclear emission has the greatest mass?
A) positron
B) gamma ray
C) beta particle
D) alpha particle
D) alpha particle
The wave-mechanical model of the atom describes the location of electrons
A) as loosely packed in the nucleus of an atom
B) as densely packed in the nucleus of an atom
C) in circular paths around the nucleus
D) in orbitals outside the nucleus
D) in orbitals outside the nucleus
Q6. Which process can be used to separate a mixture of two liquids having different boiling points?
A) deposition
B) distillation
C) filtration
D) sublimation
B) distillation
The element technetium, Tc, has several isotopes. The bright-line spectrum of technetium has been observed in the spectra of some stars.
Compare the energy of an electron in the first shell of a technetium atom to the energy of an electron in the third shell of the same atom.
An electron in the first shell of a Tc atom has less energy than an electron in the third shell.
Which electron configuration represents the electrons of a phosphorus atom in an excited state?
A) 2-8-5
B) 2-8-6
C) 2-7-6
D) 2-7-4
C) 2-7-6
ynthetic radioisotopes may be made by bombarding other nuclides with neutrons. The equations below represent a sequence of reactions converting stable iron–58 to cobalt–60, which is used in medical treatments.
State the neutron to proton ratio for an atom of the 58Fe in equation 1.

32:26
When a ground state electron in an atom moves to an excited state, the electron
A) absorbs energy as it moves to a higher energy state
B) absorbs energy as it moves to a lower energy state
C) releases energy as it moves to a higher energy state
D) releases energy as it moves to a lower energy state
A) absorbs energy as it moves to a higher energy state
Based on Table N, which particle is emitted by the radioactive decay of francium-220?
A) an alpha particle
B) a beta particle
C) a positron
D) a neutron
A) an alpha particle
State, in terms of protons and neutrons, why the various nuclides of technetium are isotopes of each other.
— Atoms of technetium have 43 protons, but can have different numbers of neutrons.
Given the diagram of a laboratory apparatus:
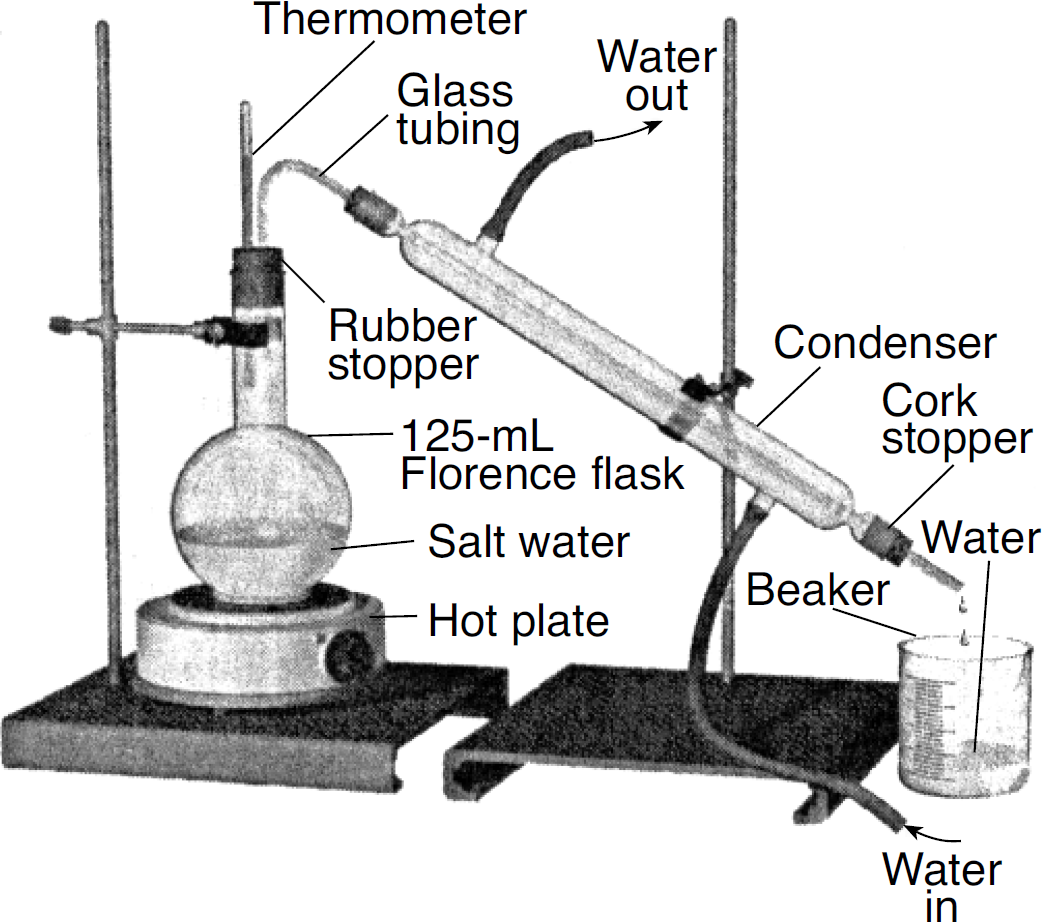
This apparatus is used for which process?
A) filtration
B) distillation
C) chromatography
D) electrolysis
B) distillation
In which type of reaction can an atom of one element be converted to an atom of another element?
Transmutation
Which statement describes a chemical property of iron?
A) Iron is malleable.
B) Iron conducts electricity.
C) Iron reacts with nitric acid.
D) Iron has a high melting point.
C) Iron reacts with nitric acid.
The atomic masses and natural abundances of the two naturally occurring isotopes of silver are shown in the table below.
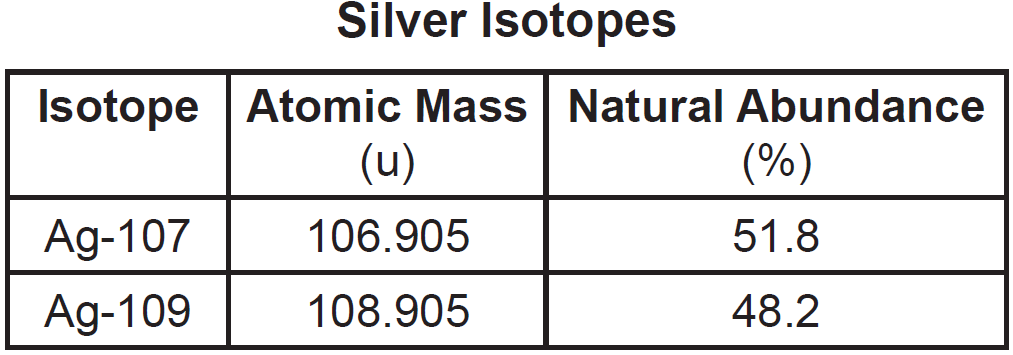
Which numerical setup can be used to calculate the atomic mass of silver?
A) (106.905 u)(51.8) + (108.905 u)(48.2)
B) (106.905 u)(51.8%) + (108.905 u)(48.2%)
C) (106.905 u)(48.2) + (108.905 u)(51.8)
D) (106.905 u)(48.2%) + (108.905 u)(51.8%)
B) (106.905 u)(51.8%) + (108.905 u)(48.2%)
Which conclusion was developed as a result of the gold foil experiment?
A) Atoms are mostly empty space.
B) All atoms are hard, indivisible spheres.
C) Atoms have different volumes.
D) All atoms have the same volume.
A) Atoms are mostly empty space.
Which term represents a nuclear reaction?
A) combustion
B) fermentation
C) transmutation
D) saponification
C) transmutation

State, in terms of elements, why equation 2 represents a transmutation reaction.
An atom of iron is changed to an atom of cobalt.