Protons, Neutrons, and electrons are the subatomic building blocks of this
What are atoms?
How many protons are in this element?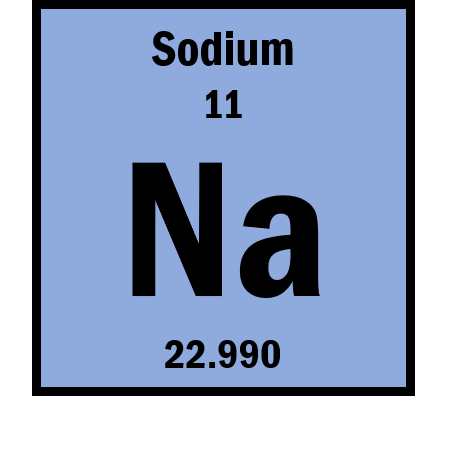
What are 11?
Altering the appearance or state of a substance without changing its chemical composition is evidence of this type of change
What is a Physical Change?
This type of heat transfer is done through physical touch.
What is conduction?
Elements that are generally dull, brittle, poor conductors of heat and electricity.
What are nonmetals?
This subatomic particle has a negative charge
What is an electron?
What group would one find the Alkaline Earth Metals?
What is group 2?
Formation of gas, color change, temperature change, or a formation of a precipitate indicate this type of change.
What is a chemical change?
This type of heating is how energy travels from the Sun to the Earth.
What is radiation?
These elements exhibit properties of both metals and nonmetals and is located along the staircase line on the periodic table.
What is a metalloid?
These two subatomic particles are found in the nucleus of an atom.
What are protons and neutrons?
How many neutrons are typically found in this element?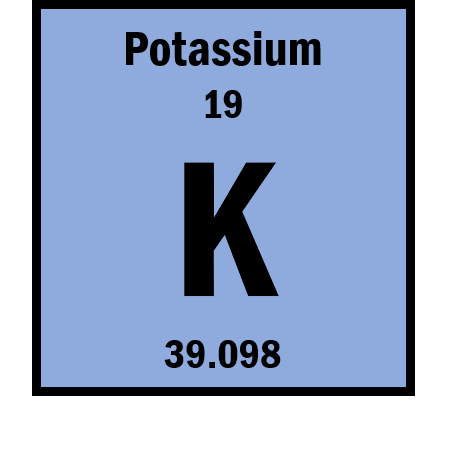
What is 20?
Melting ice to form water is an example of this type of change.
What is a physical change?
This type of heating leads to the _____________ currents of our planets asthenosphere.
What is convection?
These elements are typically good conductors of electricity.
What are metals?
This determines the identity of the element
What is the number of protons?
Name this element:
Mixing vinegar and baking soda to produce carbon dioxide gas is an example of this type of change.
What is a chemical change?
In the Earth's atmosphere, heating from the sun causes air near the Earth's surface to become warmer and less dense. This warm air rises, creating areas of lower pressure at the surface. Cooler, denser air then moves in to fill the areas of low pressure, resulting in what?
What is wind?
Silicon (Si) is an example of this type of element.
What is a metalloid?
The number of __________________ determines an atom's chemical behavior (what the atom will react with)
What is valence electrons?
Who is credited for structuring the Periodic table?
Who is Dimitri Mendeleev?
This term refers to a type of reaction in which energy is released, typically in the form of heat.
What is and exothermic reaction?
This is the type of heating an air fryer uses
What is convection?
These elements typically gain electrons in chemical reactions
What are nonmetals?