A.the tendency to attract electrons in a covalent bond
B.two-dimensional molecular geometry
C.the most stable configuration of valence electrons arranged around covalently-bonded atoms.
C
E=Octahedral
M=Square pyramidal

Hybridization=SP
ClO2-
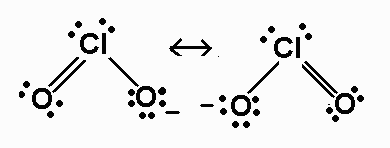

polar
The steric number refers to:
A. the number of bonded atoms plus the number of non- bonding electron pairs.
B. the number of bonded atoms
C.the number of lone pairs associated with an atom
A
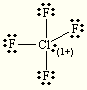
E=Trigonal Bipyramidal
M=Seesaw

Steric:6
Hybridization:sp3d2
NO2-
%20resonance%20structures.jpg)

Polar
Molecules that have a permanent dipole are found to-
A.have a higher boiling point
B.Become oriented in an electric field
C.Exhibit intramolecular attractive forces
D.have both positive and negative ends(sides)
E.All of the above
E
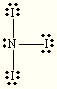
E=Tetrahedral
M=Trigonal Pyramidal

Steric:4
hybridization:sp3
CO32-
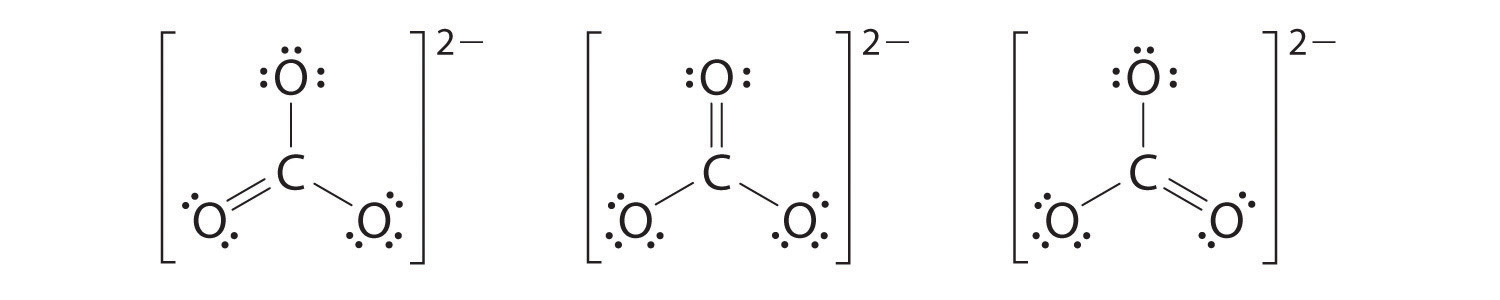
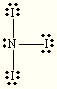
polar
An atom that has undergone sp hybridization has how many non-hybridized p-orbitals?
A.1
B.2
C.3
D.4
B
![]()
E=Trigonal Planar
M=Bent
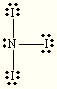
Steric:4
hybridization:sp3
BH4

non-polar
A.results from the overlap of two non-hybridized p-orbital.
B.Allows for free rotation around the bond axis
C.they involve the non-valence electrons in the 1s orbitals.
A

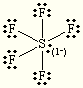
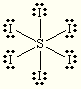
E and M= Octahedral

Steric:6
Hybrization:sp3d2

each O is +1
S is +2
![]()
Non-polar