A row on the periodic table
Period
The particle responsible for the identify of an element.
Proton
Type of matter made of only one type of atom or compound.
pure substance
The measurement for this amount with the correct number of sig figs.
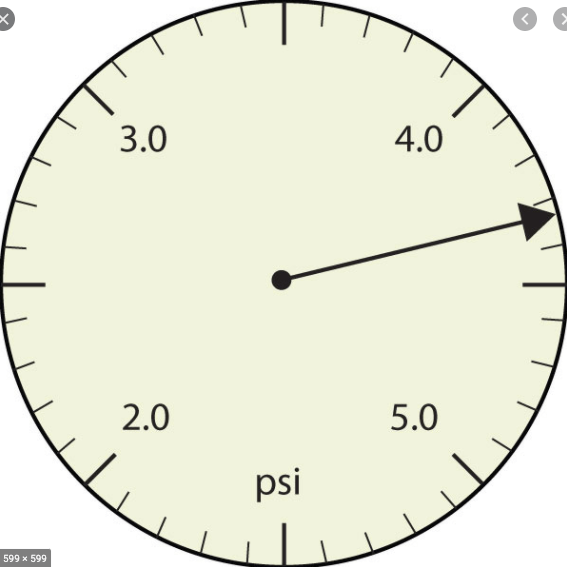
4.33 psi
Type of energy released in a nuclear reaction.
Gamma radiation
What is an orbital? How many electrons fit in one orbital?
A 3D area where electrons are likely to be found. 2 electrons can fit in one orbital (spinning in opposite directions).
Highly reactive nonmetals with 7 outer electrons
Halogens
Phosphorus, -3
Statement about all matter based on kinetic molecular theory.
(1 yard = 0.9144 meter)
3.3 meters
The largest type of particle released during radioactive decay. (in nuclear symbol notation)
42He (alpha particle)
What would the be the principal and angular quantum numbers for an electron in a 4d orbital?
P = 4, A = 2
Reactive metals with two outer electrons that may be found in their pure form in nature.
Alkaline earth metals
Hyphen notation and nuclear symbol notation for the most common isotope of the element with 74 protons.
Tungsten - 184, 18474W
Relationship between temperature and kinetic energy.
They are directly proportional.
Number of atoms in 25.0 grams of calcium.
3.76 x 1023 atoms
An isotope has a half life of 4.5 years. If you have 400. g of the sample, how much will be left after 13.5 years?
50.0 g
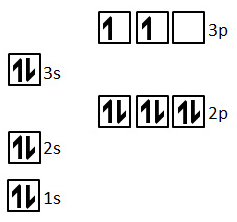
Draw the orbital diagram for Silicon (Si)
1)The trend across a period.
2) The trend down a group.
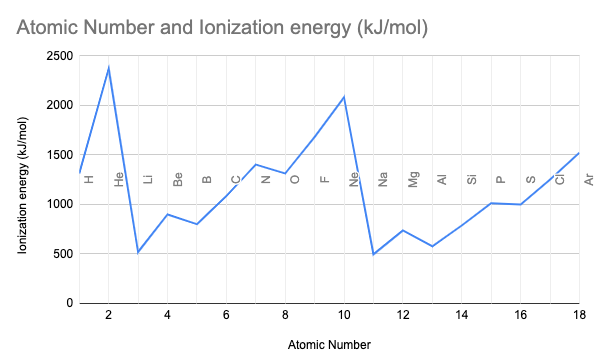
1) Increasing
2) Decreasing
What is the difference between an isotope and an ion?
Isotopes have different numbers of neutrons (different mass), while ions have different numbers of electrons (different charge)
The hydrogen molecule would be moving with a higher velocity than the water molecule.
A 30.0 L sample of nitrogen inside a rigid, metal container at 20.0 °C is placed inside an oven whose temperature is 50.0 °C. The pressure inside the container at 20.0 °C was at 3.00 atm. What is the pressure of the nitrogen after its temperature is increased to 50.0 °C?
3.31 atm
What is the process used for creating new elements larger than uranium?
Artificial Transmutation
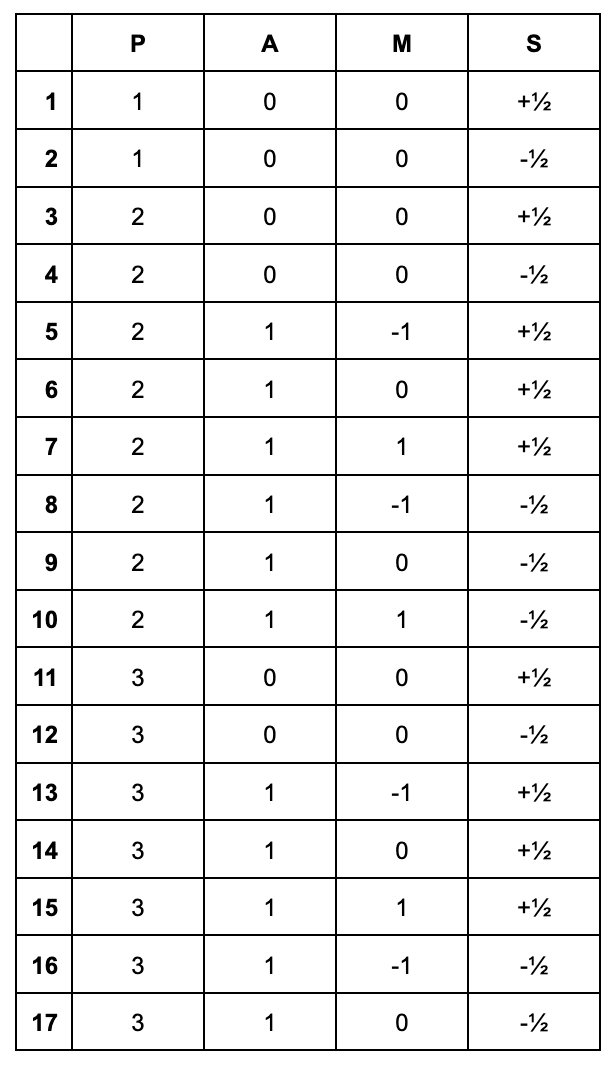
Write out the quantum numbers for all of the electrons in chlorine.
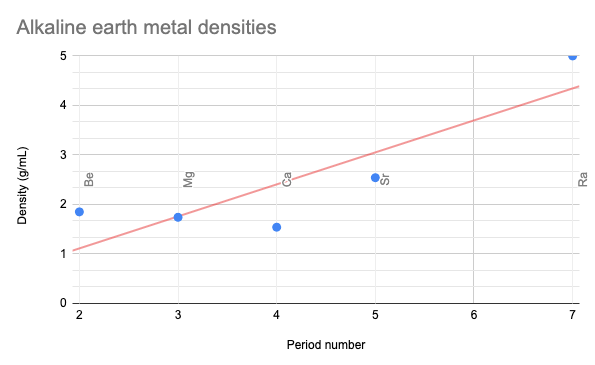 Identify the missing element and give the density based on the graph.
Identify the missing element and give the density based on the graph.
Barium, 3.6g/cm3
Draw how an atom becomes a cation. Draw how an atom becomes an anion.

Rocks can be used to store heat in solar-heated homes. If the specific heat of the rocks is 0.821 J/g-C, what temperature change would 50,000 g of rocks undergo if they emitted 450,000 J of heat?
10 degrees (1 SF)
At what temperature in Celsius, does a 48.5g sample of argon gas fill a container of 4.56L at 2.50 atm?
-159 degrees Celsius
Write an equation for the beta decay of thorium-232.
23290Th --> 0-1e + 23291Pa
Draw the orbital diagram, write the complete electron configuration, and write the noble gas configuration for plutonium (Pu)
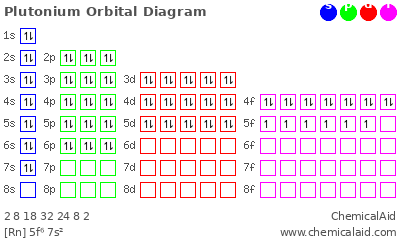
1s22s22p63s23p64s23d104p65s24d105p66s24f145d106p67s26d15f5
[Rn]7s26d15f5