Define a Rate of a Chemical Reaction
The speed at which products are formed per some unit of time
The same
N2 + 3H2 <--> 2NH3 + heat. Which way does the system shift when N2 is added?
To the right
The average kinetic energy of a substance, this controls particle movement speed
Temperature
Where electrons are located in the atom?
The electron cloud
If concentration is increased the rate of the chemical reaction will...
Increase!
The forward and reverse reactions have stopped at equilibrium, true or false?
False
N2 + 3H2 <--> 2NH3 + heat. Which way does the system shift when NH3 is removed?
To the right
The amount of a substance per some amount of volume
Concentration
BE CAREFUL: N2 + 3H2 <--> 2NH3 + heat. What direction will the reaction shift (forward or reverse) if we increase the concentration of NH3?
It will shift reverse (left) to relieve the stress.
Decreasing the temperature ______________ the rate of the reaction
Decreases
The concentrations of reactants and products are ________ at equilibrium
Constant
Define Le Chatelier's Principle
A system shifts to reduce change
A substance that increases the rate of a reaction without being used up!
A Catalyst
If a chemical system is at equilibrium, any change in the system will always result in an increase of which of the following?
The reaction that opposes the change
Decreasing the particle size increases the _____________ which increases the rate of a reaction
Surface Area
Define Equilibrium
When the forward and reverse reaction are occurring at the same time and same rate.
N2 + 3H2 <--> 2NH3 + heat. What happens to the concentration of [NH3] when heat is added to the system?
The concentration decreases
The amount of energy needed for a reaction to occur
Activation Energy
A An increase in the concentration of W or Y will increase the rate of the reverse reaction.
B An increase in the concentration of X or Z will increase the rate of the reverse reaction.
C An increase in the concentration of W or X will increase the rate of the reverse reaction.
D An increase in the concentration of Y or Z will increase the rate of the reverse reaction. 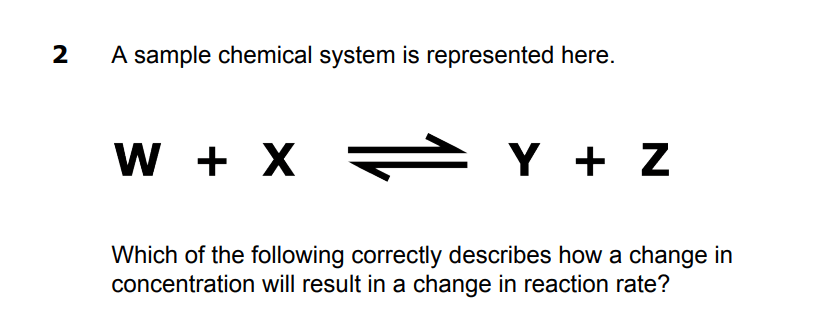
D. An increase in the concentration of Y or Z will increase the rate of the reverse reaction.
There is more product that can break down to reform reactant! it Has to shift left (reverse).
Catalysts increase the rate of a chemical reaction by...
Decreasing the activation energy
Draw a graph of concentration (y) vs time (x) for a system that has reached equilibrium
Mr. Regester will draw on the board!
N2 + 3H2 <--> 2NH3 + heat. What happens to the concentration of[N2] when the pressure is decreased?
It increases
A biological catalyst
An Enzyme
Given a reversible reaction that contains gasses, If you increase the pressure which side will be favored (increase in concentrations)? (products or reactants)
The side with fewer molecules or moles of gas.
Increasing the pressure gives the reaction less space and forces molecules to collide together--increasing the side with stuff that has been combined together in fewer molecules.