How many significant digits are in the number 1.0190?
Five
Is the following compound covalent or ionic? (NH4)2CO3
Ionic
What is the mass number of an atom of sodium with 11 protons, 12 neutrons, and 11 electrons?
23
How many grams of nitrogen are in 64 g of caffeine (C8H10N4O2)? MM = 194.19 g/mol
39.94 g N
The fact that 16.0 g of oxygen and 2.0 g of hydrogen combine to give 18.0 g of water demonstrates what natural law?
Conservation of mass
How many significant digits should this answer have?(0.24086-0.1055)/0.040
Two
What is the name of P4S10?
Tetraphosphorus decasulfide
Which pair of the following are isotopes? 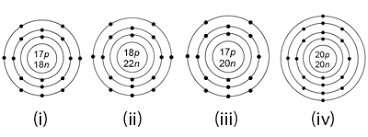
i and iii
How many atoms are in a 200-g sample of oxygen gas?
3.76*1024atoms
Given a 5 g and a 2 g block of iron, what is the relationship of the density and volume between the two? (same, larger, smaller?)
Density is the same, volume of 5 g block is larger
The density of a certain substance is 6.3 g/cm3. Aliens have landed on your planet and demand that properties be expressed in their units of density, glorps/eeks3. 1 glorp = 0.324 g, and 1 cm = 2.7 eeks. What is the density in alien units?
0.988 glorps/eeks3
What is the name of H2CO3?
Carbonic acid
In terms of subatomic particles, what do the atoms 51V, 54Fe, and 52Cr all have in common?
number of neutrons
The empirical formula of a compound is C2H6O. The molecular mass for the compound is 184.276 g/mol. What is the molecular formula for this compound?
C8H24O4
Consider the ratio of the mass of oxygen in carbon monoxide vs. carbon dioxide. What natural law is this an example of?
law of multiple proportions
Calculate the number of moles in 17.8 g of the antacid magnesium hydroxide.
0.305 moles
What is the name of the compound MnSO4?
Manganese (II) sulfate
Calculate the atomic mass of Zinc assuming the three following isotopes and abundances.
64Zn: 63.929 amu, 51.3%, 66Zn: 65.926 amu, 29.8%, 68Zn: 67.925 amu, 18.9%
65.279 AMU
An artificial sweetener is 57.14% C, 6.16% H, 9.52% N, and 27.18% O by mass. Calculate the empirical formula of the sweetener.
C28H18N2O5
What is the mass percentage of oxygen in a molecule of water, and what natural law is this an example of?
88.9%, law of constant composition
The recommended infant (0-3 months) dose of acetaminophen is 12.5 mg/kg of body mass every four hours. If acetaminophen is available in liquid form with a concentration of 160 mg/5.0 mL, how many mL should be administered to an infant weighing 8.9lbs?(1 kg = 2.2 lbs)
1.6 mL
What is the formula for tin (IV) chloride pentahydrate?
SnCl4∙5 H2O
Gallium has 2 isotopes and the average mass of 69.72 amu.Find the abundance of these isotopes(69Ga= 68.93 amu, 71Ga= 70.92 amu)
69Ga= 60.3%, 71Ga= 39.7%
The element Thallium, Tl, has two stable isotopes Tl203 and Tl205. Is the percent abundance of Tl203 or Tl205 greater? Or are they equal?
% abundance of Tl203 < % abundance of Tl205
What is the name of group 7a on the periodic table?
the halogens