Chemical Reactions
What type of reaction is the following?
2 MgO --> O2 + 2 Mg
Decomposition
What is the vocabulary word for the big numbers you put to fill in the blank to multiply all elements and balance a chemical reaction
Coefficient
What property of water is when water molecules are strongly attracted to each other?
Cohesion
What are 2 out of the 3 different levels of saturation in a solution?
Unsaturated
Saturated
Supersaturated
If a chemical formula has a capital N, then it is always insoluble.
FALSE
What are 3 of the 5 types of chemical reactions?
Synthesis
Decomposition
Single Replacement
Double Replacement
Combustion
Write down the numbers to balance out this chemical equation.
___ SiO2 + ___HF --> ___SiF4 + ___H2O
SiO2 + 4 HF --> SiF4 + 2 H2O
What are 3 of the 5 properties of water?
Cohesion
Adhesion
Universal Solvent
Polarity
Surface Tension
If a point is above a line, is it considered unsaturated, saturated, or supersaturated?
Supersaturated
Is NaI soluble or insoluble?
Soluble
Which type of reaction happens when 2 reactants combine to make 1 product?
Synthesis
Using the activity series, will the following reaction happen?
Mn + PtO --> Pt + MnO
Reaction will happen
What happens to the surface tension of water when adding soap to it?
It decreases/breaks, etc.
Which letter areas on the graph would be supersaturated for KCl?
A + B
Is CaOH soluble or insoluble?
What type of reaction is this?
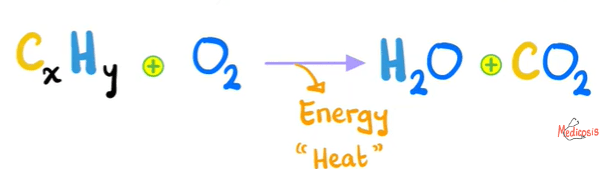
Combustion
How would you show that a chemical in a chemical reaction is dissolved in water?
Aqueous (aq)
Which property of water best explains its ability to dissovle many different salts and chemicals?
Universal Solvent
 Which chemical is the most soluble at 60 °C?
Which chemical is the most soluble at 60 °C?
Pb(NO3)2
If a chemical contains the element rubidium, will it be soluble or insoluble?
Soluble (Alkali metal)
What type of reaction is only used when using the activity series?
Single Replacement
Write down the numbers to balance out this chemical equation.
___ C9H20 + ___O2 --> ___CO2 + ___H2O
C9H20 + 14 O2 --> 9 CO2 + 10 H2O
Polarity are the different charges on water molecules. If there was a strong positive charge outside, which element in water would be more attracted to the positive charge?
The oxygen (in water it is slightly negative)
 At about what point are both NaCl and KClO3 both saturated?
At about what point are both NaCl and KClO3 both saturated?
Around 40 g & 90 °C
Label each chemical in the reaction as soluble (aq) or insoluble (s)
AgNO3 + NH4OH --> NH4NO3 + AgOH
AgNO3, NH4OH, NH4NO3 = All soluble (aq)
AgOH = Insoluble (s)