What groups of metal reacts vigorously with water and forms hydrogen gas in the process?
alkali metals and alkaline earth metals
Between tin and zinc, which replaces iron in iron sulphate?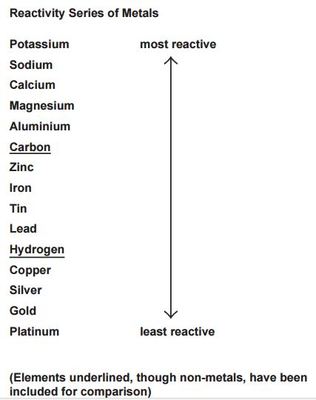
Zinc
What homologous series contain unsaturated compounds?
Alkene
Give a nonrenewable source of energy.
fossil fuel
Which is the most reactive metal in group 1?

Francium
Give a metal that can be extracted from its ore using carbon reduction.

aluminum, magnesium, calcium, sodium, potassium
What nonmetal is commonly found in steel?
carbon
How can planting trees reduce the problem with greenhouse gases?
Carbon dioxide is used during photosynthesis
Give 2 properties of metals.
lustrous, malleable, ductile, good conductors of heat and electricity, and have high boiling point
The reaction shows the reaction of lithium with water. 2Li + H2O → 2LiOH + H2
Write the balanced chemical equation for the reaction of Na with water.
2Na + 2H2O → 2NaOH + H2
How does the hardness of metals in group 1 changes as you go down the group?
decreasing down the group
What are the products when a metal reacts with hydrochloric acid (HCl)?
Metal chloride (salt) and hydrogen gas
The setup shows the extraction of aluminum. Explain why extraction of aluminum is expensive.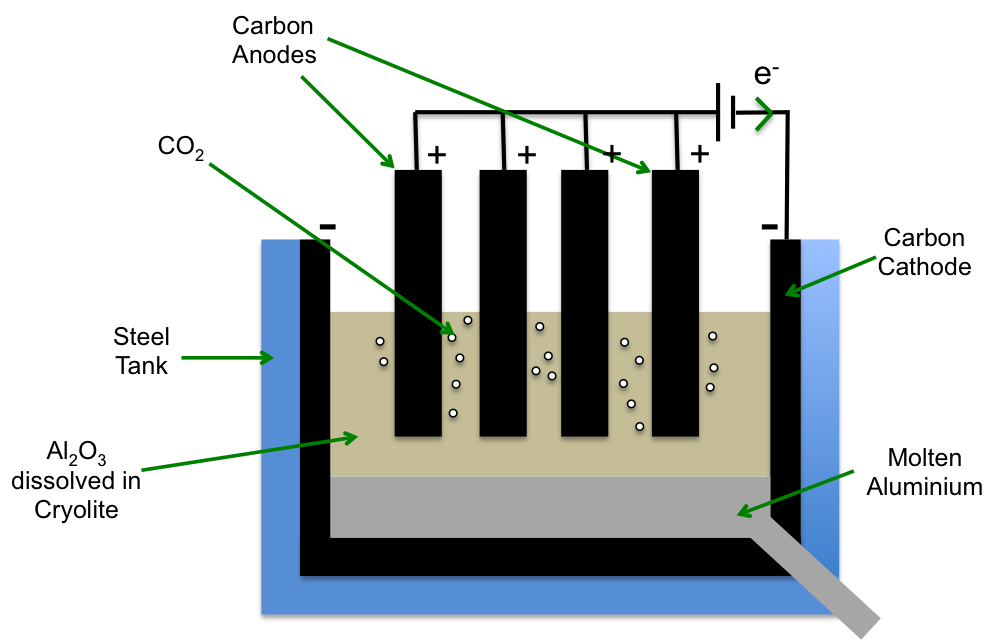
due to the frequent replacement of carbon electrodes
Why do we dissolve aluminum oxide in molten cryolite?
to reduce the overall melting point of the mixture
What is used as anode in the extraction of aluminum setup?
Graphite
What are the two substances that contributes to rusting of iron?
water and oxygen
What is sacrificial method to prevent rusting and how does it work?
Iron is in contact with a more reactive metal, such as zinc. Zinc gets oxidized more readily instead of iron so iron will not rust.
Balance the chemical equation below.
____Fe + 3 O2 → ___Fe2O3
4 Fe + 3 O2 → 2 Fe2O3
What process produces ammonia?
Haber process
What process produces sulfuric acid?
contact process
What is the importance airlock in the fermentation process? 
prevents air from entering your fermentation vessel while allowing the CO2 made during fermentation to escape
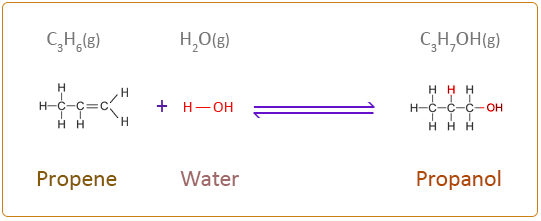
What do you call the reaction where water is reacted with alkene to produce alcohol?
Hydration reaction
What hydrocarbon will you mix with water to produce ethanol?
ethene
Why does boiling point of alkane and alkene increases as you add more carbon?
Intermolecular forces of alkenes and alkanes gets stronger with increase in the size of the molecules.
Draw the structural equation of the reaction between propene and water.