Which step of the scientific method is missing:
1.Ask a question
2. ?????????????????????
3. Design Experiment
4. Collect Data
5. Analyze Data
6. ?????????????????????
7. Communicate Results
2. Make Hypothesis
6. Form Conclusions
Number of protons (and electrons if neutral atom)
What charge and mass does an electron have?
Negative charge, negligible mass
What types of elements are in Ionic compounds?
Metal & Nonmetal (Opposite charges)
What type of reaction is this:
2Na + Cl2 >> 2NaCl
Synthesis
Rust, Burning, Explosion (any type of reaction)
Which particle has the weakest penetrating power?
a. Beta
b. Gamma
c. Positron
d. Alpha
D. Alpha
7
What is the name of the following compound:
C2Cl4
Dicarbon tetrachloride
How would you balance the following equation:
___BaCl2 + ___NaBr >> ___CaCl2 + ___BaBr2
___BaCl2 + __2_NaBr >> ___CaCl2 + ___BaBr2
Which is an EXTENSIVE property?
a. Color is blue
b. Density is 2g/mL
c. Mass is 5g
d. Condutive
c. Mass is 5g
(Extensive = changes with amountIntensive = stays the same no matter how much)
What is the difference between Nitrogen-14 and Nitrogen-15?
Different mass, different isotope, and different number of neutrons
Which atom is larger? (use a periodic table)
O vs. S
Sulfur
What is the shape of the following compound: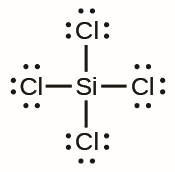
Tetrahedral
How could you write this equation in words:
CaCO3 + 2HCl >> CaCl2 + CO2 + H2O
Calcium carbonate plus hydrogen chloride yields calcium chloride, carbon dioxide, and water.
Which particle diagram shows a mixture? 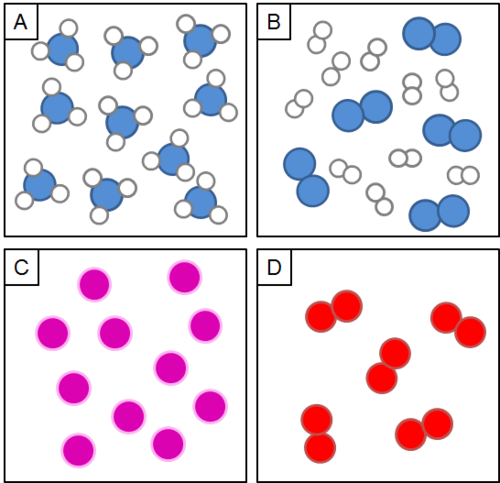
B.
Gold Foil Experiment = Nucleus
Cathode Ray Experiment = Electrons & Protons
Why does atomic radius decrease across the periodic table?
Nuclear Charge (more protons in the nucleus)
What is the formula for Magnesium Hydroxide?
Mg(OH)2
When will double replacement be no reaction?
If all products are (aq) soluble
Biochemistry is the study of________________.
the chemical processes in living things
List 5 models of the atom.
Billiard Ball (Dalton), Plum Pudding (JJ Thompson), Rutherford Model, Bohr Model, Quantum/Electron Cloud Model
What is the short hand electron configuration of silver? (Use a periodic table)
[Kr]5s24d10
What is the criss-cross method and how do you use it?
Determine the ions of each element in the compound, then criss-cross the charges and make them subscripts in the compound.
Is AgCl soluble or insoluble in water?
Insolube