They have particular spaces they can orbit and these orbits are
Electron Cloud
Atomic Radius is
the size of an atom
There are three main types of nuclear radiation?
Alpha, Beta, Gamma
Sn2+
Tin (II) Ion
all non-zeros are
significant
First cloud holds
2 electrons
Why does the Atomic Radius decreases left to right across a period it's because of ?
More Protons
When a neutron decays it becomes a protein and an electron..
Beta Particle
Li1+
Lithium Ion
0's to the right of a nonzero with NO decimal
may be significant
Second cloud holds
8 electrons
Ionization Energy is
energy required to remove an electron
travels faster than alpha and beta particles.
gamma rays
Sr2+
Strontium Ion
0's to the left are
not significant
third cloud holds
18 electrons
Ionization increases left to right because ___?
Decreases going down because of __?
Protons have a stronger hold
Shielding effects
slow moving and doesn't travel well through matter
Alpha particle
Pb4+
Lead (IV) Ion
Round the following to 3 significant figures
6.070
6.07
fourth cloud holds
32 electrons
Electronegativity is
an electrons attractiveness level
What is the symbol for Alpha, Beta and Gamma Particles?
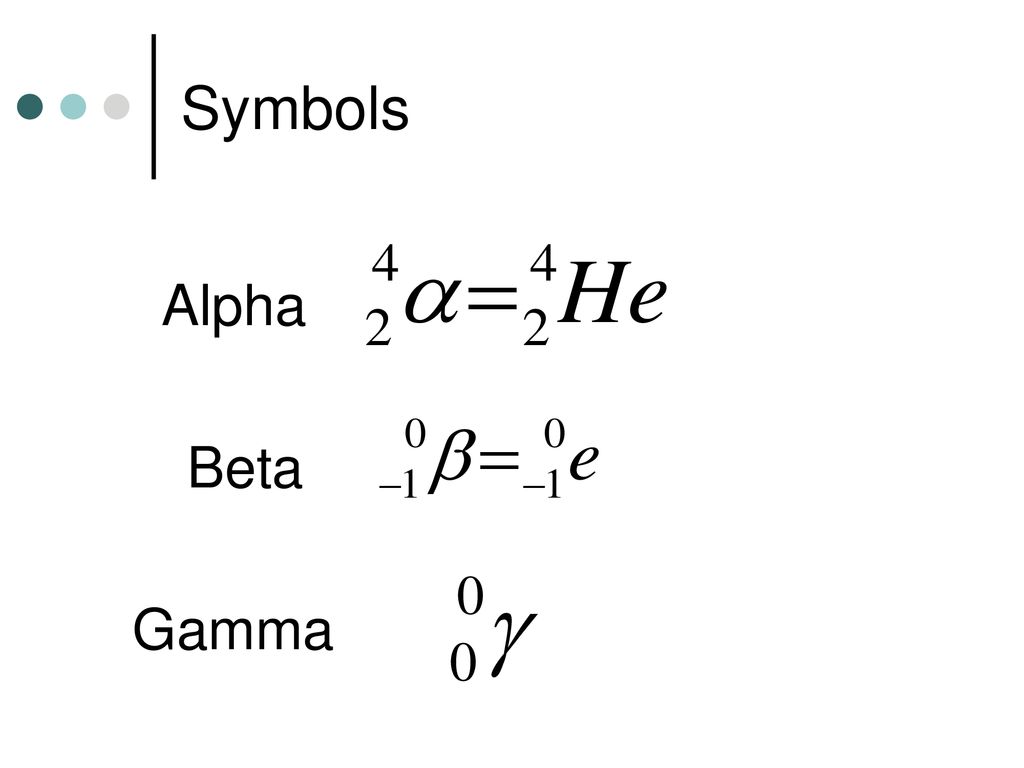
Ca2+
Calcium Ion
Round the following to 3 significant figures
20.52
20.5