How do the particles move in a solid object?
They vibrate in place
What is the name of the phase change from a liquid to a gas?
Evaporation
A block has a mass of 20g and a volume of 15cm3. What is it's density?
1.33g/cm3
What is density?
A measure of how tightly packed materials are in a given space (mass per volume).
Why does ice float on water?
It is less dense as a solid than a liquid (one of the only materials like this!)
Draw a diagram of what the particles look like in a solid, liquid, and a gas
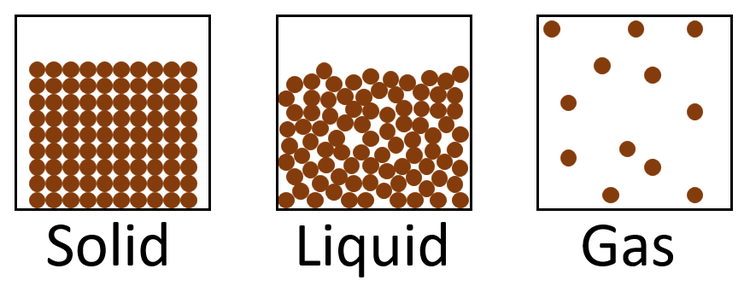
What is the name of the phase change from a gas to a solid?
Deposition
A metal cube measures 3cm on all sides. It has a mass of 250g. What is it's density?
9.26g/cm3
Liquid A has a density of 1.2g/mL and liquid B has a density of 1.7g/mL and do not mix. Which would go to the bottom of a container?
Liquid B
What is the phase change from solid to liquid?
Melting
Why do gasses have a non-definite shape?
They are moving so fast that they spread out over the entire area of the container they are in.
A goldsmith pours liquid gold into a mold and cools it into a ring. Which phase change is this an example of?
Solidification
A rubber duck has a volume of 50mL and a density of 0.68g/mL. What is it's mass?
34g
Vinegar has a density of 0.9g/mL, water has a density of 1.0g/mL, rubbing alcohol has a density of 0.8g/mL and honey has a density of 1.4g/mL.
How would you expect these liquids to layer?Alcohol on top, then vinegar, then water, then honey on the bottom
Droplets of water forming on a mirror after a hot shower is an example of....
Condensation
How does the motion, energy, and space between the particles change as you heat a liquid?
Particles gain energy, so they begin to move faster, separate from each other. Some will gain enough energy to become a gas.
Frost forming on a window is an example of which phase change?
Deposition (warm air inside cools directly into a solid when it touches a very cold window).
A mystery liquid has a density of 1.7g/mL and this volume. What is the mass?:max_bytes(150000):strip_icc()/meniscus05-58b5b2e45f9b586046bb2365.png)
35.7g (would also accept 36.55g)
Why does the density of a metal change as you heat it? Does it become more or less dense?
The particles in the metal gain energy from the heating. This causes them to move faster and further apart, and the material expands. This means the volume increased. Increased volume = decreased density.
How does the attraction of particles to one another change as they move from a solid to a liquid to a gas?
With each transition they become less attracted to each other (most attracted as solids, least attracted as gasses)
Why does a puddle eventually dry up even if it is not boiling?
The outer particles are exposed to warm air. This will eventually give the surface layer of particles enough energy to move faster and become a gas and evaporate. Then this repeats with the next layer, until all the particles become a gas.
Particles slow down a lot.
A lot of energy is lost.
Particles move close together, and begin to vibrate in place.
A mystery cube has a mass of 6047g and a density of 17.2g/mL. What is it's volume?
351.57mL
Vinegar has a density of 0.9g/mL, water has a density of 1.0g/mL, rubbing alcohol has a density of 0.8g/mL and honey has a density of 1.4g/mL.
You drop an iron nail (7.87g/mL) and a grape (1.1g/mL) into a beaker with these liquids. In which liquid will these objects sit in?
Iron nail will go the bottom of the beaker as it is the most dense out of everything.
The grape will sit somewhere near the top of the honey layer (it is less dense than honey but more dense than water).
Why does stirring help a solid dissolve faster?
Stirring gives the solid particles energy, and breaks them up and causes them to spread apart from each other. This helps them get enough energy to become a liquid faster and dissolve.