These particles have a positive electrical charge.
What are Protons?
Oxygen is an example of this.

What is an element?
This is the numeric range of the pH scale.
What is 0 to 14?
This occurs when two or more molecules interact to form new molecules.
What is a chemical change?
This scientist proposed that atoms are hard indestructable spheres.
Who is Dalton?
These subatomic particles have a negative electrical charge.
What are electrons?
Water (pictured below) is an example of this.
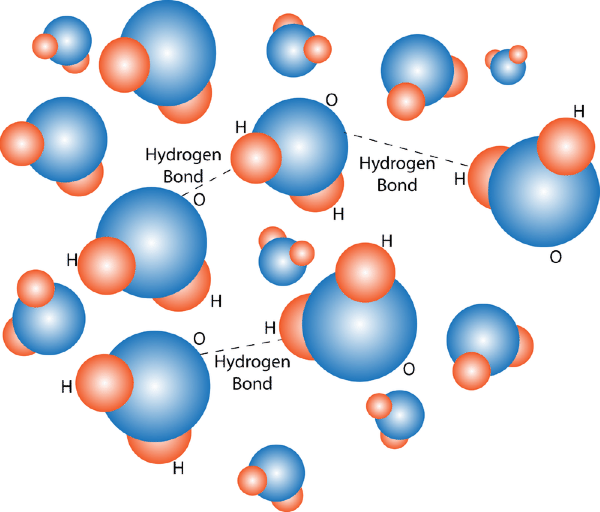
What is a compound?
This is the pH of a neutral substance, like water.
What is 7?
This occurs with a change to the appearance of a substance, but no new molecules are formed.
What is a physical change?
This scientist ran an experiment with gold and found that atoms contain mostly empty space but have a dense core.
Who is Rutherford?
This subatomic particle has no electrical charge.
What are neutrons?
Salt water is an example of this.
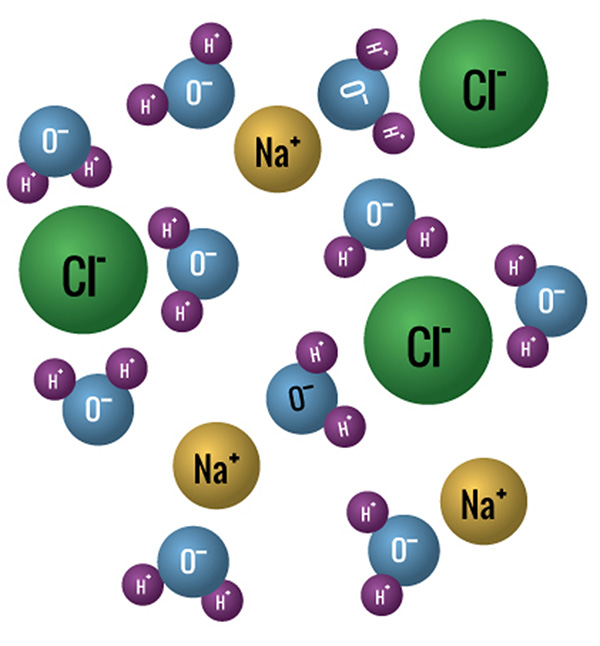
What is a mixture?
These are two physical properties that are associated with a base.
What is bitter and slippery?
A change in shape, texture, or state of matter are all evidence of this kind of change.
What is a physical change?
This scientist discovered negatively charged particles within neutral elements, then came up with the plum pudding model as an explanation.
Who is Thompson?
To get atomic mass, you need to add these two particles.
What are protons and neutrons?
Salt, Water, Iron Sulfide, and Sodium Chloride are all examples of this.
What are compounds?
pH is short for this term.
What is the Power of Hydrogen?
The release of a gas (bubbles), presence of a fire, or the formation of a precipitate are all evidence of this kind of change.
What is a chemical change?
This scientist proposed the atomic model commonly used in chemistry classes today to depict the subatomic particles of a given element. His model showed electrons in orbitals.
Who is Bohr?
Each different element has a consistent amount of these, which gives the element its atomic number.
What are protons?
This is a series of letters and numbers that represent Elements or Compounds in chemistry.
What is a chemical formula?
An acid has this chemical property.
What is reacting to metals?
What are the two types of evidence that can occur with a physical or chemical change, but they depend on whether or the change is expected.
What is a change in color or temperature?
He created a version of the periodic table of elements and predicted properties of elements that had not been discovered yet.
Who is Mendeleev?