Which of these describes a chemical change?
a. Frost disappears from a window in the morning.
b. A decrease in temperature reduces the volume of a gas.
c. Soot is formed as a candle burns.
d. A cup of hot tea cools down.
c. Soot is formed as a candle burns.
Which of these represents a physical change?
a. Iron rusting
b. Ice melting
c. Wood burning
d. Food spoiling
b. Ice melting
Which of these is an intensive property ?
A Volume
B Mass
C Boiling point
D Number of moles
C Boiling point
Which of these are composed of two or more different substances that are chemically combined in a definite ratio?
A Compounds
B Mixtures
C Elements
D Solutions
A Compounds
A substance has a definite volume and a definite shape. Which state is it in?
a. solid
b. liquid
c. gas
d. liquid or solid
a. solid
Which of the following is an example of a chemical change?
a. Combustion of gasoline
b. An apple being bitten
c. An ice cube being swallowed
d. Absorption of a water molecule
a. Combustion of gasoline
Which of the following is an example of a chemical change?
a. Ice cracking
b. Sugar dissolving
c. Milk souring
d. Lead melting
c. Milk souring
Which of the following is an extensive property of sodium chloride?
A Mass of 15 g
B Density of 2.17 g/cm3.
C White color
D Solubility in water
A Mass of 15 g
An unknown silvery powder has a constant melting point and does not chemically or physically separate into other substances. The unknown substance can be classified as —
A an element
B a compound
C a mixture
D an alloy
A an element
A substance does not have a definite volume or shape, it is easily compressed, and it is fluid. Which state is it in?
a. solid
b. liquid
c. gas
d. liquid or gas
c. gas
Which of the following processes is an example of a physical change associated with an oak tree?
a. Decomposition of bark by bracket fungi
b. Starches and sugars being broken down during energy production
c. Water and carbon dioxide being converted to glucose
d. Evaporation of water from the surfaces of leaves
d. Evaporation of water from the surfaces of leaves
Which of the following is the best evidence that digestion involves chemical change?
a. Time is required to accomplish results.
b. The products’ mass equals the reactants’ mass.
c. Different compounds are involved.
d. New substances are produced.
d. New substances are produced.
Which of the following is an extensive property of iron?
A black color
B mass of 25 g
C density of 7.86 g/cm3
D magnetic
B mass of 25 g
The picture shows a model of the element —
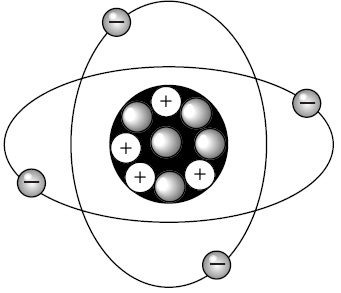
A fluorine
B helium
C beryllium
D oxygen
C beryllium
Which does NOT describe the liquid state of matter?
a. The liquid takes on the shape of its container.
b. The liquid has a definite volume and an indefinite shape.
c. The particles in a liquid can easily move past each other.
d. The liquid has a definite volume and a definite shape.
d. The liquid has a definite volume and a definite shape.
A sample liquid is cooled from 150°C to 30°C, causing the liquid to change into a solid. Which of the following has occurred?
a. Chemical composition has changed.
b. A physical change has occurred.
c. A new compound has formed.
d. Evaporation of a solvent has occurred.
b. A physical change has occurred.
When a cookie is eaten, what part of the digestive process causes a mainly physical change?
a. The action of enzymes
b. Saliva breaking down starches
c. Chewing and swallowing
d. The action of stomach and intestines
c. Chewing and swallowing
Which represents an intensive property of the metal?
A It has a mass of 22.43 g.
B It measures 10.4 cm.
C The volume is calculated to be 3.56 cm3.
D The density is calculated to be 6.30 g/cm3.
D The density is calculated to be 6.30 g/cm3.
When copper and oxygen chemically unite, they form —
A an ion
B a gas
C a compound
D a mixture
C a compound
Which state(s) of matter describe(s) a fluid?
a. gases and liquids
b. liquids and solids
c. gases only
d. liquids only
gases and liquids
Rust (Fe2O3) forms on an iron (Fe) pipe after prolonged exposure to humid air. What type of change does this illustrate?
a. Mechanical
b. Nuclear
c. Chemical
d. Physical
c. Chemical
Which process best demonstrates a chemical change in distilled water?
a. Freezing the water
b. Separating the water into its elements
c. Calculating the water’s density
d. Dissolving sugar in the water
b. Separating the water into its elements
Which of the following is an intensive property of a sugar solution?
A volume
B density
C sweetness
D mass
B density
The experiment illustrates that iron and sulfur combine to form —
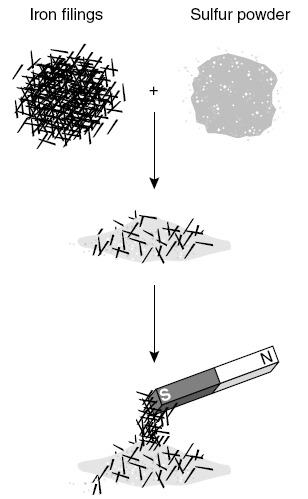
A a nonmetal
B a compound
C a mixture
D an alloy
C a mixture
How are solids and liquids alike?
a. Each has a definite volume.
b. Each has an indefinite shape.
c. Each has an indefinite volume and indefinite shape.
d. Each has a definite volume and definite shape.
a. Each has a definite volume.