Atoms and Models
Periodic Table
Elements, Compounds, and Mixtures
Physical/ Chemical Properties and Changes
Density
100
At the center of an atom is the nucleus. What 2 subatomic particles are found there?
Protons and Neutrons
100
What are the rows (horizontal) called on the periodic table?
Periods
100
This cannot be broken down any more. It is as simple as it can get.
Element
100
If a substance has the ability to rust, is that a physical or chemical property?
Chemical. Rusting is the reaction to Oxygen.
100
What is density?
It is the mass of a substance per unit of volume
200
What are the names and the charges of each of the subatomic particles in an atom?
Protons = Positive
Electrons= Negative
Neutrons= Neutral
200
Which of these pairs of elements have the most in common and how do you know?
1. K and Ca
2. O and S
O and S
Oxygen and Sulfur because they are in the same group.
200
Ocean water is an example of what? BE SPECIFIC
Homogeneous Mixture
200
You can test these properties out without changing the identity of the substance.
Physical Properites
200
What are the units for measuring density?
grams/ cm3
grams/ mL
300
Which element is this a model of?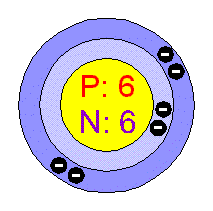

Atomic number 6
Carbon
300
The "Zig-zag" line seperates what two things on the periodic table?
Metals on the left
Nonmetals on the right
300
Tell me which ones are pure substances and which are not pure substances.
Element= Pure
Compound= Pure
Mixture= Not Pure
300
Name 3 ways to know a chemical change has taken place.
Production of a gas
Production of a precipitate: a solid
Change in smell
Change in temperature
Change in color
300
A metal block has a volume of 10 mL and a mass of 30 grams. What is the density of the block?
30g/ 10mL = 3g/mL
400
Use the picture to tell me how many protons, neutrons and electrons are in an atom of Phosphorus

15 protons
15 electrons
16 neutrons
400
What kind of bond would Nitrogen and Sulfur make?
Will the two atoms share electrons or transfer electrons?
nonmetal to nonmetal =Covalent bond
Share electrons
400
Which letters are showing compounds?

A,D,I = Pure Compounds only
Incorrect:
B,C,G = Mixtures
E,F,H = Elements
400
If I melt aluminum, I am testing what kind of property?
Physical property
400
The mass of a substance is 50g. The density is 2g/mL what is the volume of the substance. MUST include UNITS
25mL
500
Electrons are located in where in the atom?
Electron cloud
500
Name 3 properties of a metal.
Conductors
Malleable
Ductile
High Density
Luster
500
How can you separate a mixture?
Physically. Examples: Filtering/distillation
500
If a substance has the ability to burn this is what kind of property?
Chemical Property
500
What is the term called when you use water to find out the volume of something?
Displacement