Which bond is the strongest?
Triple bond
What type of compound is KBr?
Ionic
Nonpolar
What type of bond is formed between atoms of similar electronegativities?
Covalent bonds
What type of bond is CO2?
Covalent
What is the chemical formula for an ionic compound?
Formula unit
What type of bond is a iron alloy?
Metallic
What is a crystal lattice?
An orderly arrangement of ions
Draw the Lewis structure of H2O. Is it polar or nonpolar?
It is polar
Atoms bond because...
2nd law of thermodynamics says that systems move towards a state of minimum energy
Atoms are most stable at the lowest energy level (is easier when they are compact)
Name a bond that is 100% ionic.
Doesn't exist
Give two properties of covalent compounds.
Poor conductors of heat and electricity
Forms network covalent substance
Draw the Lewis structure of O2. Is it polar or nonpolar?
It is nonpolar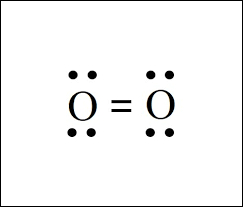
Are there single, double, or triple bonds in C2? Is it polar or nonpolar?
Double bonds
It's nonpolar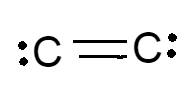
What are delocalized electrons?
Electrons that are collectively shared among metal atoms.
Give three characteristics of ionic compounds.
Dense, brittle, and hard solids
High melting points
Poor conductors of electricity as solids
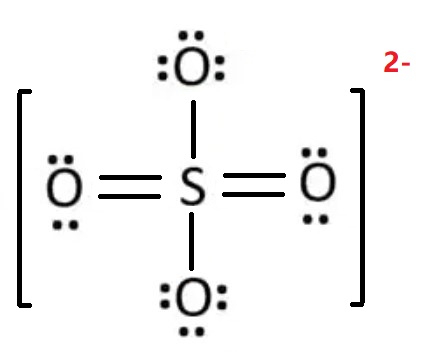
Draw the Lewis structure of sulfate (SO42-).
Give four characteristics of metallic compounds
Forms alloys
Lustrous
Malleable
Ductile
Name the seven diatomic elements.
H2
N2
O2
F2
Cl2
Br2
I2