How many Joules are in 1 cal?
What is the formula for change in energy?
1 cal = 4.184 J
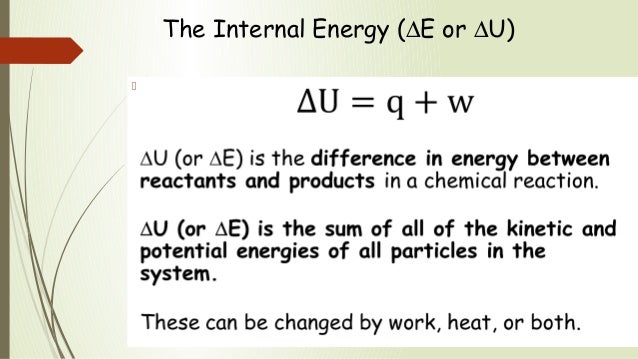
ΔU = q + W
ΔU = Ufinal - Uinitial
Work in a system is negative for exothermic reactions, such as explosions.
True or False?
True.
- Work = exothermic
+ Work = endothermic
Since ΔU isolated system = 0, ΔU system = -ΔU surroundings and energy is conserved.
What law of thermodynamics is applied?
First law.
What points represent the transition from liquid to gas? At what point does the substance fully change into a liquid?
Transition: D-E
Liquid: C
Classify each example as either endothermic or exothermic.
a) An explosion
b) Frost forming on a window
c) Melting ice cubes
a) Exothermic
b) Exothermic
c) Endothermic
:max_bytes(150000):strip_icc()/endothermic-and-exothermic-reactions-602105_final-c4fdc462eb654ed09b542da86fd447e2.png)
How much heat is generated when 3.21 moles of O2 are burned?
CH4(g) + 2O2(g) → CO2(g) + 2H2O(g)
change heat degrees= -802 kJ/mol
-2.57x103 kJ
A sample of methanol gas is ignited in the presence of oxygen inside a closed film canister.
As the reaction occurs, 140 J of heat are lost to the surroundings.
The expanding gas does 15 J of work to pop the top off the canister.
What is the change in internal energy, ΔE, for the system?
155 J
Hydrogen peroxide decomposes according to the following thermochemical reaction:
H2O2(l) → H2O(l) + 1/2 O2(g); ΔH = -98.2 kJ
Calculate the change in enthalpy, ΔH, when 1.00 g of hydrogen peroxide decomposes (in kJ).
-2.89 kJ
Substance A, with a weight of 15.4g and a specific heat of 2.75 J/g°C with an initial temperature of 21.2°C, has been mixed with substance B, the temperature of substance A has increased to 40.0°C. What is the heat energy (in J) substance A obtains from substance B?
796.18 J