The mass of a single proton or neutron
What is 1 amu?
The charge on a potassium atom when it loses an electron
what is +1?
The increase in hydronium ion concentration represented by a decrease of one pH unit.
LiOH, for example (be specific!)
What is an Arrhenius base?
What are melting, vaporizing (evaporating), and sublimation?
An element that has atoms with 2 electron shells and 7 valence electrons
What is fluorine?
The name of the negative ion that forms when LiOH disassociates in solution
What is hydroxide?
The pH of an aqueous solution with hydroxide ion concentrations 100 times greater than an aqueous solution with a pH of 8
What is pH of 10?
HNO3, for example (be specific!)
What is an Arrhenius base?
A property that is conserved during all chemical reactions. (multiple answers)
What is charge? What is mass? What is energy?
What all the elements in group 2 have in common that makes them have similar chemical properties
What is the number of valence electrons?
A property that is used to determine the degree of polarity of a covalent bond between two atoms.
What is electronegativity?
The pH of a 0.1 M HCl solution
What is 1?
The laboratory procedure done to determine the concentration of an unknow acid or base
What is a titration?
A type of reaction that yields salt and water as the only products
What is a neutralization reaction?
Electron that have less energy than electrons in the second shell
What are electrons in the first shell?
When a metal and a non-metal are chemically combined in fixed proportions, like NaBr for example.
What are ionic compounds?
The molarity of an acid if 20 mL are needed to neutralize 10 mL of 6 M base.
What is 3 M?
The color of bromcresol green in a basic solution
what is blue?
A stress that will cause equilibrium to shift in such a way that more phosphorus trichloride will be produced by the decomposition reaction shown below.
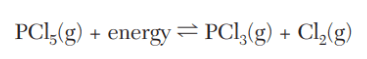
What is an increase of heat?
What is an increase of concentration of PCl5?
What is a decrease in concentration of PCl3 or Cl2?
A property that generally decreases as the elements in period 2 are considered from left to right.
What is atomic radius?
The reason electrolytes can conduct electricity
What are freely moving charged particles (ions)?
The number of moles carbon dioxide produced when 2 moles of butane react completely given the balanced equation below.
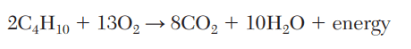
What is 8 moles?
The compound acting as a Bronstead-Lowry acid in the reaction shown below.

What is water?
What is ammonium?
The chemical formula for the salt formed when Ca(OH)2 and HBr undergo a neutralization reaction
What is CaBr2?