What is the instrument below called, and what's it used to measure?
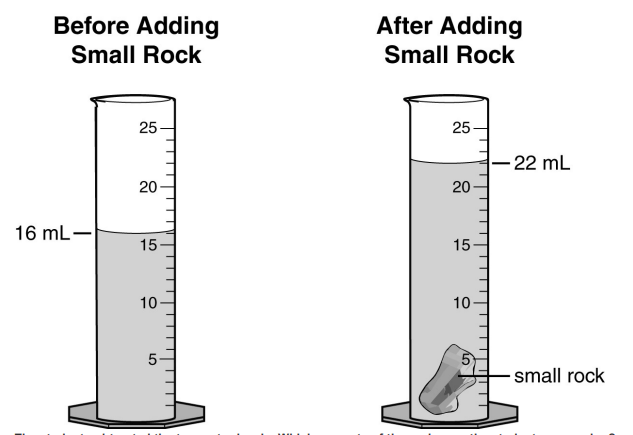
A. Beaker, volume
B. Graduated cylinder, volume
C. Triple beam balance, mass
D. Triple beam balance, density
B. Graduated cylinder, volume
Can be measured without changing the identity of the substance.
What is a physical property?
List 3 signs of a chemical change
Temperature Change
Formation of a precipitate
Color Change
Change in Smell
Formation of a gas
a rectangle is 3 cm long
Extensive property
He is the "Father of the Periodic Table"
Who is Dmitri Mendeleev
Elements in the same group have : (2 correct answers)
A. Similar chemical properties
B. Similar names
C. Same number of outer (valence) electrons)
D. The same number of protons
A. Similar chemical properties
C. Same number of outer (valence) electrons)
What's the instrument below called, and what's it used to measure?
A. Beaker, volume
B. Graduated cylinder, volume
C. Triple beam balance, mass
D. Triple beam balance, density
C. Triple beam balance, mass
Double or nothing, what units does it use to measure?
Indicates how a substance reacts with other substances; are only observed when a substance undergoes a chemical change.
What is a Chemical Property?
The type of change that occurs below is...
H2O (solid) --> H2O (gas)
Physical change
The boiling point of water is 100ºC
Intensive property
Which of the following is a physical property that Mendeleev used to design the periodic table?
A. Reactivity with metals
B. Oxidation state
C. Atomic weight
D. Crystalline structure
C. Atomic weight
Name an element with similar properties to those of sodium (Na)?
What is Li, K, Rb, Cs, or Fr
A crayon has a mass of 15g and volume of 5 ml. What is the density?
3 grams/ml
Give a defintion of:
1. Physical change
2. Physical property
3. Chemical change
4. Chemical property 
Physical change - physically changing substance
Physical prop- Can be observed w/out changing substance
Chem change - changing what substance is
Chem prop - only observed during chem rxn
Changes that includes breaking, boiling, ripping.
What are examples of physical changes?
A group of students was asked to determine the extensive properties of an object. What did the students observe as the extensive property of the object?
A. reactivity producing color
B. conductivity
C. mass
D. reactivity with acid
C. mass
What was observed that led to arrange sixty-three already-known elements by increasing atomic weights in the periodic table?
A. repetition occurs in chemical and physical properties.
B. An atomic weight corresponds to the existing abundance.
C. The number of neutrons and protons increased by one.
D. The chemical compounds formed by the elements increased by one.
A. repetition occurs in chemical and physical properties.
Which of the following will have similar properties?
A. O, S, Se
B. O, F, Ne
C. N, Ne, Na
D. H, K, P
A. O, S, Se
If the mass of the object below is 60 grams, what is its density?
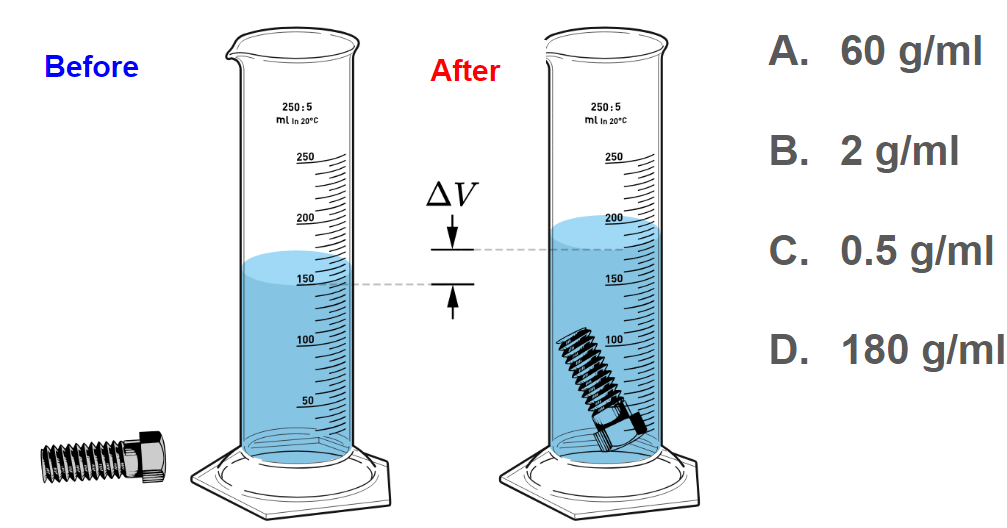
B. 2 g/ml
Phenolphthalein underwent a color change in the presence of _____.
A. Potassium anion
B. Potassium cation
C. Iodide anion
D. Iodide cation
B. Potassium cation
Double or nothing: was this a chemical or physical change?
Changes that include rusting, burning, corrosion, digestion, decaying, respiration.
What are examples of chemical changes?

Mass = 3.8 grams
In 1913, English physicist Henry Moseley used X-rays to measure the wavelengths of elements to find their number of protons. He then rearranged the PT to what we see today - based on increasing:
A. atomic mass B. atomic number C. number of neutrons D. abundance found in nature.
B. increasing atomic number
Find an element with similar chemical properties to Barium (Ba).
A. Ca an Ra, because they are in the same group and have similar chemical properties.
B. Cs and La, because they are in the same period number.
C. Ca and Y, because they are 90 degree angle and have similar properties.
A. Ca an Ra, because they are in the same group and have similar chemical properties.
What could be the unknown "Property X"?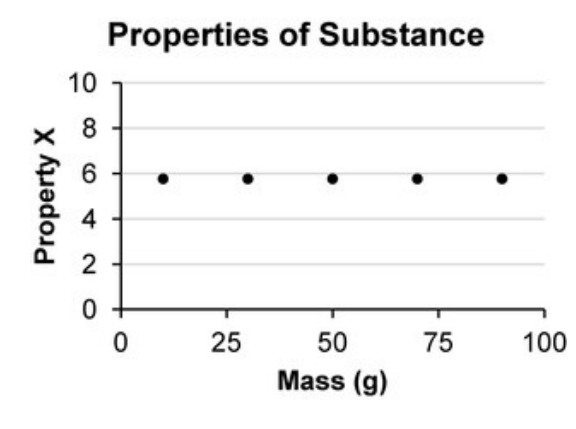
A. Volume
B. Length
C. Weight
D. Density
D. Density
Triple or nothing: is this an extensive or intensive property, and explain how you know.
When you heat copper sulfate crystals, the water evaporates leaving behind anhydrous copper sulfate which is white in color.
Is the appearance of the copper residue a physical or chemical property? Explain.
Physical property you can observe the color without creating a new substance
Which statement describes the type of change and the chemical properties of the product and reactants?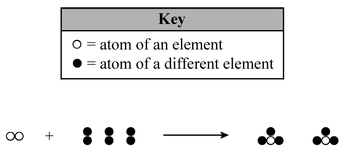
A. The equation represents a chemical change, with the product and reactants having identical chemical properties.
B. The equation represents a physical change, with the product and reactants having identical chemical properties.
C. The equation represents a chemical change, with the product and reactants having different chemical properties.
D. The equation represents a physical change, with the product and reactants having different chemical properties.
C. The equation represents a chemical change, with the product and reactants having different chemical properties.
Keisha measured mass and another property, Y, of three pure samples of the same compound. She recorded her data in the table below:
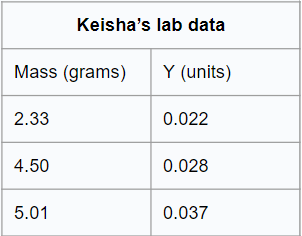
Which of the following best describes property Y?
A. Y is an intensive property because it varies directly with the size of the sample.
B. Y is an extensive property because it does not vary with the size of the sample.
C. Y is an intensive property because it does not vary with the size of the sample.
D. Y is an extensive property because it varies directly with the size of the sample.
D. Y is an extensive property because it varies directly with the size of the sample.
In 1869, Mendeleev created a periodic table in which elements were ordered by atomic weight and placed in groups based on their chemical properties. Mendeleev's decision to design the periodic table in this way allowed immediate advances in chemistry by —
A. Providing a framework for models of electron configurations.
B. discovering the inert gas family, the noble gases
C. providing an explanation for the reactive properties of the alkali metals.
D. predicting the physical/chemical properties of yet undiscovered elements
D. predicting the physical/chemical properties of yet undiscovered elements
Which sequence of atomic numbers represents elements that have similar chemical properties?
A. 3, 12, 21, 40
B. 4, 20, 38, 88
C. 9, 16, 33, 50
D. 19, 23, 30, 36
B. 4, 20, 38, 88
