This type of reaction occurs when a hydrocarbon reacts with oxygen.
Combustion reaction
The point on a phase diagram where the solid state, the liquid state, and the gas vapor state can coexist is___?
The triple point
What are the units for molarity (M)?
mol/L
Convert 142 nutritional Calories to calories. (1000 cal= 1 Cal)
142,000
N₂ + 3H₂ → 2NH₃
What is the mole ratio of H2:NH3?
3:2
Predict the products of this synthesis reaction:
2Na + Cl2→
2NaCl
P1V1=P2V2 (T is constant)
V1= 10 L P1= 175 kPa P2= 350kPa V2= ?
5 L
Calculate the molarity of 1.6 L of a solution containing 0.013 mol of KBr?
0.0081 M
If the temperature of ethanol increases from 25 °C to 78.8 °C, what does ΔT equal?
53.8 °C
You expect to make 10 g of product, but only make 5 g of product. What is the percent yield of the reaction?
(5/10)*100
50%
Balance this synthesis reaction and give the sum of coefficients:
___C + ___S → ___CS2
C + 2 S → CS2
1+2+1 = 4
What is the Ideal Gas Law Equation?
PV=nRT
What volume of a 3.0 M KI stock solution would you use to make 0.3 L of a 1.25 M KI solution?
0.125 L
q=mcΔT ; How much heat is absorbed if:
mass=34.4 g
change in temperature= 53.8 °C
specific heat = 2.44 J/g°C
4,516 J
How many atoms are in 3 moles of He?
1.81 × 1024atoms
What type of reaction is this:
Cu + 2AgNO₃→ Cu(NO₃)₂ + 2Ag
PV=nRT
P= 2.5 atm V= 12 L T= 298 K
R= 0.0821 L*atm/K*mol n=??
1.23 mol
You have 1500 g of a bleach solution. The % by mass sodium hypochlorite (NaOCl) is 3.62%. How many grams of NaOCl are in the solution?
54.3 g
What is the definition of enthalpy?
The heat (q) content of a system at constant pressure.
How many grams of O2 are in 20 moles?
(molar mass = 32 g/mol)
640 grams
What type of reaction is this:
2KClO₃ → 2KCl + 3O₂
Decomposition
According to Charles's Law, volume and temperature are _________ proportional.
Directly
Name at least 1 substance were the solubility decreases with temperature?
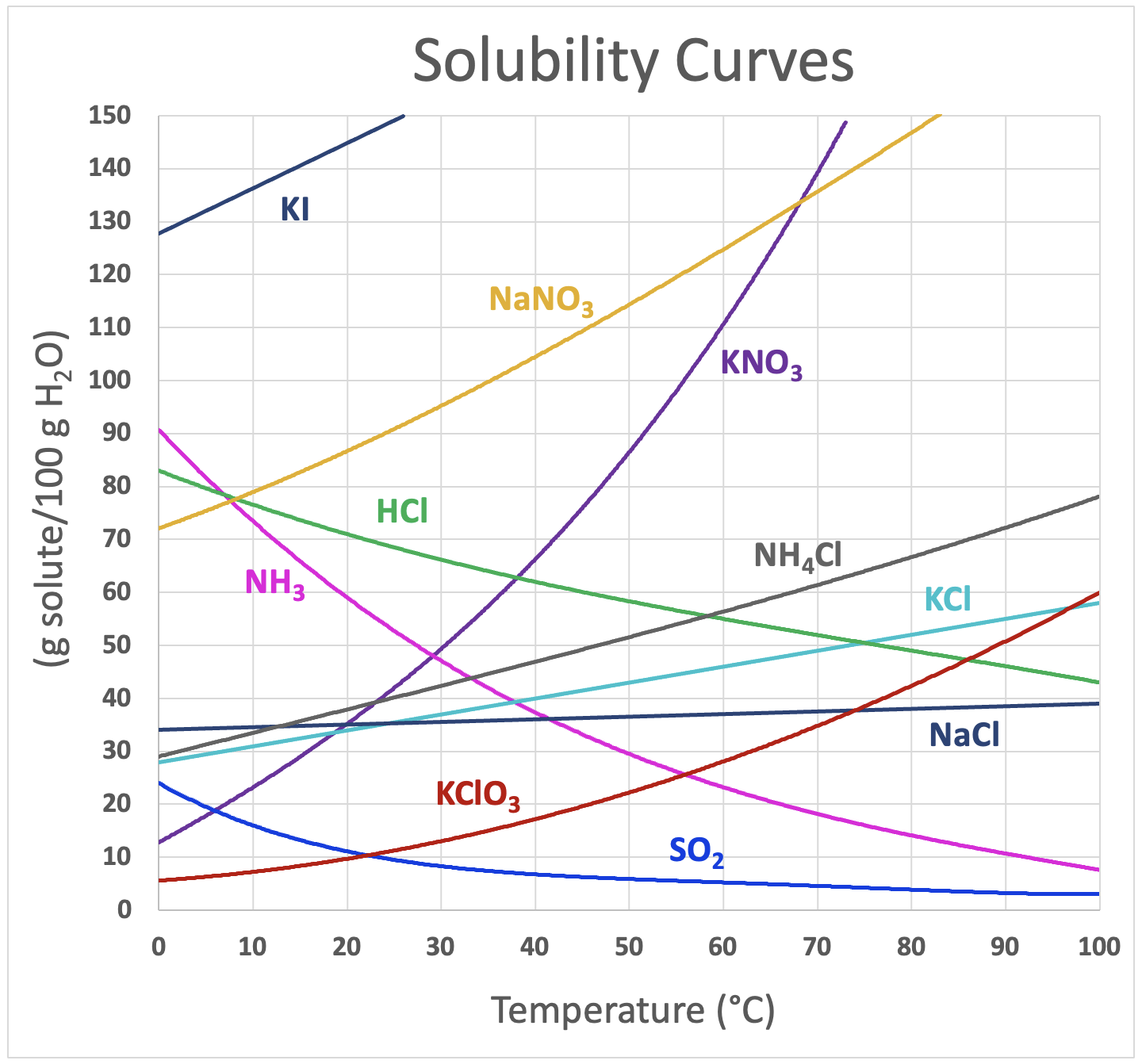
NH3 ; SO2 ; HCl
q=mcΔT; What is the specific heat if: mass=90g heat = 25.6 J Change in temperature = 1.18 °C
0.241 J/g°C
How many moles are in 58.5 grams of NaCl?
1 mole