What quantity is measure in g/mol?
Molar Mass
The subatomic particle with a mass of nearly 'zero'.
What is the electron?
The number of dots on a the Lewis Dot Diagram of a neutral carbon atom.
What is 4 dots, :C:?
In general, salts dissolve better in _________ temperatures.
What is 'higher'?
the summer solstice
During a reaction, what is the name of the reactant that 'runs out' first?
Limiting Reactant
The subatomic particle that gives an element it's identity.
What is a proton?
The number of electrons that sodium tends to gain or lose in a chemical reaction:
dotNa
What is 'lose 1 electron'?
The molarity of a solution in which 0.5 moles are dissolved in 0.25 L of solution.
What is 2M, "Two Molar"?
Which planet has the most moons?
Jupiter
After a reaction, what is the name for the reactant that is 'left over'?
XS (excess) reactant
The number of neutrons present in this isotope of oxygen:
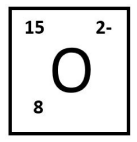
What is 7 neutrons?
The option below that correctly models Lithium Oxide.
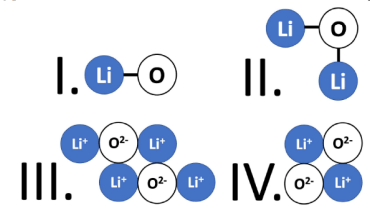
What is Option III?
The name for the acid, HCl.
What is hydrochloric acid?
What is the longest running American animated TV show
Simpsons
How many moles of oxygen (O2) are in 16 g of oxygen gas?
0.5 moles
The (neutral) element that is represented by this Bohr model:
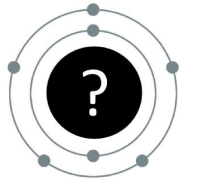
What is Nitrogen?
The geometry (shape) of a Boron Trifluoride molecule:
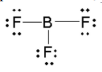
What is trigonal planar?
Adding salt to water tends to ________ the freezing point of the water.
What is lower?
Area 51 is located in which state?
Nevada
2H2 + O2 -> 2H2O
Ionization energy describes the energy required to remove this subatomic particle from an atom.
What is the electron?
The number of bonding electrons are pictured in the Lewis structure below:
What are 6 bonding electrons?
Adding solute tends to ______ vapor pressure by inhibiting evaporation, while have no effect on condensation at the liquid-gas boundary.
What is reduce?
Which members of the Beatles are still alive?
Paul and Ringo