Give the definition of an ion.
A single particle with either a positive or negative charge.
Which element has the atomic number 9?
Fluorine
What type of radiation causes the largest decrease in the mass number of an element?
Alpha Radiation
How many electrons can be found within a single orbital?
2
What forms in an acid and base reaction?
Water and salt
If the formula for an ionic compound is represented by A2B3, what is the charge of the A ion?
+3
How many electrons does this neutral element have?
79
Beta decay occurs when...
Greater mass number than in the periodic table
How many valence electrons are found in the element nitrogen?
5
What is the following type of reaction?
AgNO3 ----> Cu(NO3)2 + Ag
Single-Replacement
A chemical change produces a _______.
How many neutrons does a neutral Ruthenium atom have?
57
Complete the nuclear equation
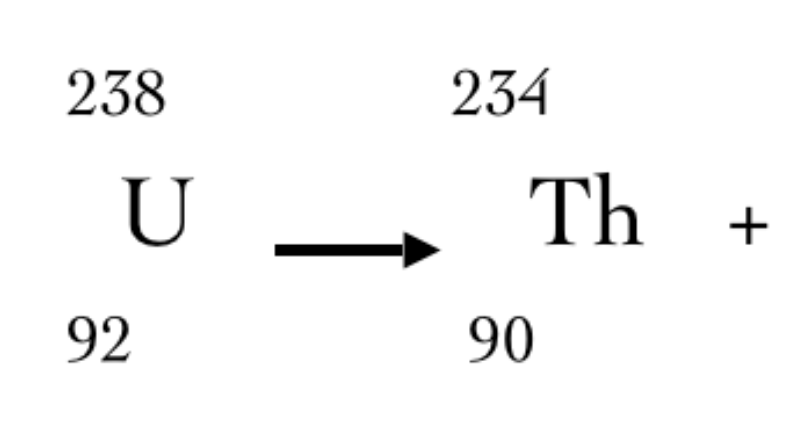
42He
Which element has the highest ionization energy?
Li, K, Cs, or Rb
Lithium
What is the following type of reaction?
C3H8 + 5O2 ----> 3CO2 + 4H2O
Combustion
Density is an example of a ________.
Physical Property
The sum of protons and neutrons in an atom equals the ________.
Mass Number
What happens to the nucleus during gamma decay?
Nothing, it is unchanged
Which element has the following electron configuration?
1s22s22p63s23p2
Silicon
Balance the following chemical equation.
__TiCl4 + __Na ---> __NaCl + __Ti
1TiCl4 + 4Na ---> 4NaCl + 1Ti
What is the name for SnCO3?
tin(II) carbonate
The nucleus of an atom is ________ charged.
Positively
Alpha decay occurs when...
Atomic number is equal to or greater than 84
All of the elements in group 6A will end in the sub level abbreviation of ______.
p4
Balance the following chemical equation.
__C3H8 + __O2 ---> __CO2 + __H2O
1C3H8 + 5O2 ---> 3CO2 + 4H2O
