What is Avogadro's number?
The name of the compound K2O
What is potassium oxide?
To balance a chemical equation, these may be added or adjusted.
What are coefficients?
The mole ratio of nitrogen to ammonia
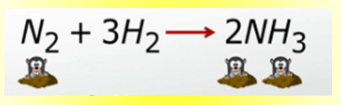
What is 1:2?
This type of substance taste sour and feels tingly on your skin.
What is Acid?
atm, torr, Pa, kPa are all units for this variable that affects gases
What is pressure?
Category is Solutions
Make your Wagers
The Molarity of a NaCl solution that has 4.0 moles of NaCl and 3500 mL total solution.
What is 1.1 M?
One mole of H2O has a mass of _____ grams.
What is 18.0 g?
The formula for dichlorine monoxide
What is Cl2O?
The generic equation represents this type of chemical reaction.
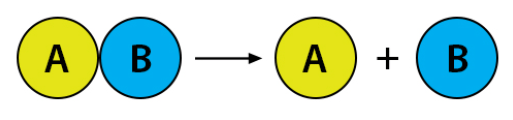
What is decomposition?
In a chemical reaction, the reactant that is all used up is called the _____.
What is limiting reactant?
This type of substance turns phenolphthalein pink.
What is a Base?
To convert from Celsius to Kelvin you must do this
What is add 273?
The Molar Mass of CaCl2.
What is 111.1 g/mol? (Ca = 40.1 + Cl = 35.5 x 2 =71.0)
The formula for tetraphosphorus decasulfide
What is P4S10?
The products for this single replacement reaction:
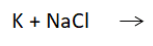
What are Na and KCl?
The measured amount of a product obtained from a chemical reaction.
What is actual yield?
The name for HCl
What is hydrochloric acid?
If the pressure of a gas goes up, then this happens to the volume,
What is decrease?
The % composition of of oxygen in Fe2O3
What is 30.1% ? (48.0/159.6 * 100)
The name for the compound Al(NO3)3
What is aluminum nitrate?
The type of chemical reaction where carbon dioxide and water are always the main products.
What is combustion?
Ammonia, NH3, is widely used as a fertilizer and in many household cleaners. How many moles of ammonia are produced when 6 mol of hydrogen gas react with an excess of nitrogen gas?
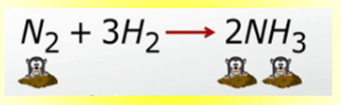
What is 4 mol of NH3?
6 mol H2 * 2 mol NH3 / 3 mol H2
H2SO4 is this type of acid
What is strong acid?
The gas law equation that allows you to solve for moles.
What is Ideal Gas Law or PV=nRT?
The number of molecules in 1 mole of water.
What is 6.02 x 1023 ?
The name for the compound FeCl3
What is iron (III) chloride or ferric chloride?
The precipitate formed from the double replacement reaction below.

What is AgBr?
The % Yield from this reaction.

What is 79%?
The procedure to determine the concentration of an unknown acid or base using a solution of known concentration.
What is titration?
The gas that would effuse faster between H2 and O2 according to Graham's Law of Effusion.
What is H2?