What is the main element in the atmosphere (78%) that we breathe? (It's not Oxygen)
Nitrogen
What type of bond shares electrons?
Covalent
A simple sugar that contains 3-10 carbon atoms
(Think Greek for 'one')
Monosaccharide
This compound is necessary for life in certain quantities, can be found in animals and plants, can be consumed, and is also made into soaps and candles.
Fat!
This is a really BIG molecules, made up of amino acids.
Protein
Macronutrients
Which of these can form covalent bonds:
Metals, Non-metals, Both Metals & Non-metals, None of these
Non-metals
Most fruits are high in monosaccharides called this:
fructose
The name chemists give to fats.
Lipids
In humans and many animals, this protein is very important in converting food into energy.
Insulin
What are covalent compounds that contains carbon atoms called?
Organic Compounds
What kind of bonds do each of these pairs of electrons have, and which is the strongest?
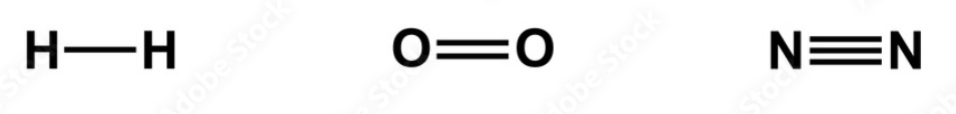
Sinlge bond Double bond Triple Bond
A compound made when two monosaccharides bond together, as well as a compound made when more than two monosaccharides bond together
Disaccharide
Polysaccharide
This lipid is made from fatty acids that have no double bond between carbon atoms
Saturated fats
These small molecules all contain carbon, hydrogen, oxygen, and nitrogen atoms. (A few also have sulfur atoms)
Amino Acids
Which of the following is/are organic:
CH2O MgC4H6O2 NO2 C12H22O11
CH2O & C12H22O11
The name for a line of carbon atoms in the middle of an organic molecule.
The carbon backbone
The name for monosaccharides, disaccharides, and polysaccharides.
Carbohydrates
This lipid is made from fatty acids that have at least one double bond between carbon atoms
Unsaturated fat
The name for the specific order of amino acids in a protein.
Primary structure
A measurement that tells how much energy your body can make from things being consumed.
Calories
Define what isomers are. You must clearly state: what is the same, and what is different, and what that produces.
Compounds with the same chemical formula but different arrangements of atoms, which lead to different properties.
This polysaccharide is found in nature, and made by plants linking many glucose molecules together.
Starch.
Bonus- are polysaccharides sweet to taste?
Name the process where double bonds are removed in an unsaturated fat to make it into a saturated fat?
Hydrogenation
Deoxyribonucleic acid
(Dee-ox-ee-rye-bo-new-clay-ik)