This classification of chemicals has properties such as : malleability, luster, conductivity and being ductile.
What are metals?
Which element is the most electronegative element on the periodic table?
What is Florine?
What element has the smallest radius? (barring Hydrogen & Helium)
What is an Florine?
Horizontal rows on the periodic table.
What are periods?
Simplest form of matter.
What are elements?
Has characteristics/properties that overlap with the other two classifications of matter
What are metalloids?
the distance from the nucleus to the outermost electron of an atom
What is atomic radius?
If our Ionization energy is increasing what are our chances of making a positive ion?
It will be less likely.
Vertical columns on periodic table.
What are groups?
The study of matter, the changes matter undergoes and the energry associated with those changes.
What is chemistry?
Not malleable, not ductile, has no luster and poorly conducts electricity.
What are non metals?
Energy required to REMOVE one electron from an atom of an element.
What is ionization energy?
If element X has a large Radius for its Period does what would you expect its ionization energy to be? what about its electronegatvivity?
Low ionization energy, High electronegativity.
Elements with similar properties; group 1, 2, 17 and 18
What are families (also known as groups)?
Why do atoms become ions?
To achieve an octet!
Located along the "staircase" except for Aluminum (Al)
What are metalloids?
What do all of the highlighted elements have in common
They all have 7 valence electrons
As we travel down this group we witness our ionization energy decrease. why?
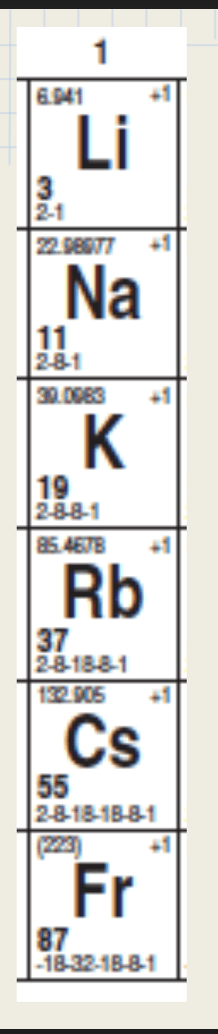
What is shielding
What is this groups name?
:max_bytes(150000):strip_icc()/alkalineearth-56a12cd75f9b58b7d0bcca7e.png)
What are Alkaline earth metals?
Consisting of two or more elements. Can only be changed by chemical means. H2O is an example of this.
What are compounds?
Located under/to the left of the staircase, except for Hydrogen (H).
What are metals?
As you move across a period what trends do we see regarding electronegativity, Ionization energy and Atomic radius?
2X points if you can tell me why
Electronegativity & ionization energy increase.
Atomic radius decreases.
Why: Increasing nuclear charge.
Correctly identify which arrow corresponds with each periodic trend.
Electronegativity, Ionization energy & Atomic radius
one trend per an arrow color
Orange & Blue: Electronegativity & Ionization Energy
Red: Radius
Group 18 is this:
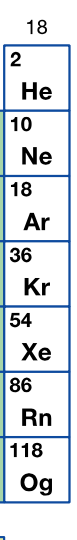
What are noble gases?
Surround the nucleus, electrons live here.
What is a shell?