What happens to ionic compounds when they dissolve?
They split apart into their individual positive and negative ions.
This definition of acids and bases specifically involves the transfer of electron pairs.
Lewis acids and bases.
To which side of the reaction does the equilibrium shift if something is added to one side of the equation?
Away from the side with more stuff and towards the side with less stuff.
Define specific heat.
The tendency for a substance to resist a change in temperature.
Which charge do GROUP 2 ions develop, and how do you know?
+2. Group 2 atoms lose their 2 valence electrons and become +2 ions.
Will ionic sodium chloride dissolve better in ethanol or water, and why?
Sodium chloride will dissolve better in water because water is more polar than ethanol.
Which definition of acids is shown by the equation below?
This is an Arrhenius acid because of the free H+ ion in the products.
Which direction would the equilibrium shift if I added ammonia to the equation below?
The reaction would shift towards the reactants.
Two objects of equal mass and identical material are touched together. Object 1 has a starting temperature of 500C and object 2 has a starting temperature of 100oC. In which direction will the heat flow?
The heat will flow from object 2 to object 1.
Describe the relationship between the strength of the LDFs between atoms and the atomic radii of those same two atoms.
If the LDF is stronger, the atomic radius must be larger.
Which molecule will dissolve best in a nonpolar solvent, A or B.
Which is the most acidic element: A, B, C, or D?
D: This carbon accepts an electron pair.
Pressure measures the amount of gas. Suppose chemical "C" in the equilibrium equation below is a gas. If I increase the pressure above the reaction vessel, which side of the reaction would be favored?
The reactants would be favored because, by increasing the pressure, we increased the amount of product.
Is the reaction below endothermic or exothermic?

EXOTHERMIC. The products are at a lower energy than the reactants, thus energy must be released during the reaction.
Describe the relationship between atomic radius and strength of ionic bonds if the charges on the compounds' ions are all equal?
In a solution of ethanol and water, what is the strongest intermolecular force between water molecules and ethanol molecules?
HYDROGEN BOND. Both molecules meet the conditions for a hydrogen bond.
Water, in red, acts as 2 types of bases in the reaction below. Name them.
Lewis
Bronsted Lowry
The reaction below is endothermic. Assume it occurs at equilibrium. Which direction would the reaction proceed if we increase the temperature.
The reaction will move toward the products because heat is a reactant.
Does "cold" exist? Why or why not?
No. We only have lack of heat.
Observe the diagram below. Why does the potential energy increase if the atoms move closer together than their most stable distance?
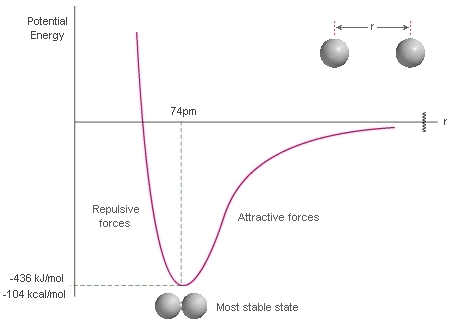
The electron clouds overlap and the atoms start to repel one another and slow down.