This law states matter cannot be created or destroyed
Law of Conservation of Mass
The distance of a wave from crest to crest
Wavelength
Atoms with all electrons paired in their orbitals are referred to as
Diamagnetic
CuO is a (ionic or covalent) compound?
Ionic
Electronegativity increases as you go (up or down) and (left or right)
Up and Right
The type of mixture in which all components are uniformly distributed
Homogenous mixture
The number of times a point on a wave passes a fixed reference point in one second
Frequency
What element does this electron configuration represent:
[Ne]3s23p2
Silicon (Si)
Chemical formula for sodium sulfite
Na2SO3
What is the molecular shape around the central atom:
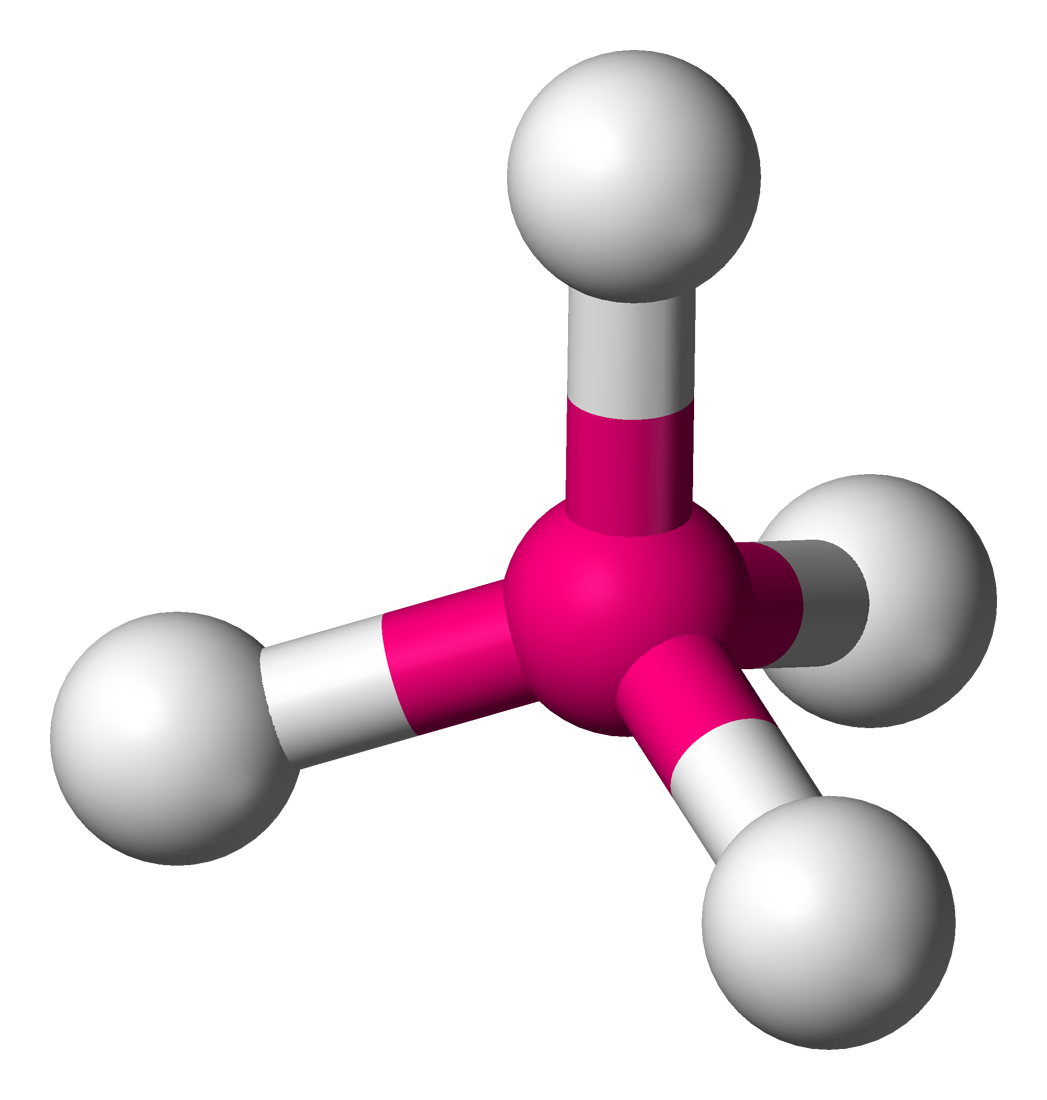
Tetrahedral
Which of the following is a heterogeneous mixture:
a) soil
b) salt water
c) air
d) coffee
Soil
Value of Planck's constant (units included)
6.626x10-34J-s
Ionization energy increases as you go (up or down) and (left or right)
Up and Right
How grams are of F are in 425g CaF2?
207g
What are the ANGLES around the central atom?

90 degrees