True or False? Valence electrons refer to electrons in the innermost energy levels.
False: valence electrons refer to electrons in the OUTERMOST energy levels of the atom.
This refers to a substance that is made of one or more elements in fixed proportions.
Compound.
Correctly identify the groups that are in each color.
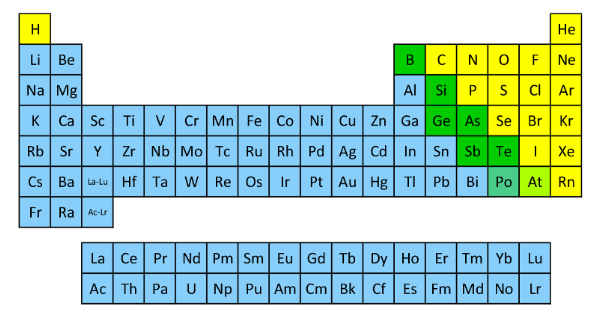
Metals = blue
Non-metals = yellow
Metalloids = green
This describes a property that a substance naturally has. It can be observed by simply observing the substance. You do not need to alter/react the substance with other substances in order to discover it.
A physical property.
JK, this one has to do with science. (Somewhat). In the television series the Magic School Bus, what was the name of Ms. Frizzle's pet lizard?
Liz
What do we call those circular things that the electrons live in?
Energy levels / shells
State the three subatomic particles in the atom and each of their corresponding charges.
1.) Proton -- positive charge
2.) Electron -- negative charge
3.) Neutron -- neutral charge
If a substance can be separated by physical processes, then it must be this ____________________
A mixture
Unlike non-metals, which are dull, this word means shiny and is often applied to metals.
Lustrous/Luster
Is density a physical property or a chemical property?
Physical Property. -- You can directly observe it. Just stick it in some water and see if sinks (high density) or floats (low density).
What is the only mammal that lays eggs?
The duck-billed platypus

How many valence electrons does the first energy level ideally want? How many valence electrons do all other energy levels ideally want?
First energy level: 2
All other energy levels: 8
True or False? All atoms have a neutral (0) charge, but a nucleus with a positive charge.
True
True or False: Elements are made up of many atoms, but the same type of atom.
True.
This class of elements reacts by LOSING electrons to form positive ions.
Metals
Is atomic radius a physical property or a chemical property?
Physical property. (You can directly observe it. You'd need an electron microscope, but you can still directly observe it).
If you add the year that the titanic sank to the year that the great Depression started, what number would you get?
(Recommend using paper and a pencil to check the math. No calculator allowed).
1912 + 1929 = 3841
What is Mr. Bloom's favorite TV show?
The office
True or false? The number of protons in an atom is equal to the number of neutrons in an atom.
FALSE: the # of PROTONS = # of ELECTRONS
# of protons can, but is not always = # of neutrons
This word refers to a fundamental substance that cannot be broken down into simpler substances by ordinary chemical means.
An element.
This class of substances is brittle, has a low density, and tends to react by pulling electrons in.
Non-metals
In table salt (NaCl), the metal (Na) loses an electron, giving it to the non-metal (Cl), which gains an electron. Is the property that metals tend to lose electrons while non-metals tend to gain electrons a chemical property or a physical property?
Chemical Property. (Describing how it reacts with other substances).
Yesterday was 9/11, one of the historic events that the US always remembers. What historic event on 12/7 is also remembered?
Pearl Harbor
An ion (an atom with a charge) has the following subatomic particles:
1.) 5 electrons
2.) 8 protons
3.) 7 neutrons.
What is the charge of this ion?
+3
The atomic number of an atom is equal to _________ while the atomic mass of an atom is equal to ________________
Atomic # = # of protons
Atomic mass = # of protons + # of neutrons
Correctly identify which group of substances the following belong too:
1.) Air
2.) Water
3.) Carbon Dioxide
4.) Oxygen
1.) Mixture
2.) Compound
3.) Compound
4.) Element
This specific group of non-metals has a special name and is characterized by very high stability and little reactivity with other elements, due to their OUTERMOST energy levels being completely filled.
Noble Gases
We talked about physical and chemical PROPERTIES, but we did not yet talk about physical and chemical CHANGES. Based on your knowledge of physical/chemical PROPERTIES, take an educated guess whether each of the following are physical or chemical CHANGES.
You must get ALL of these correct to get the points.
1.) Liquid water freezes to solid ice.
2.) An acid reacts with water and loses some hydrogen ions.
3.) A copper penny is run over by a car and gets more stretched out/flattened.
4.) A marshmallow burns in a fire and turns black.
1.) Physical-- it's still water.
2.) Chemical -- acid chemically changed by losing ions.
3.) Physical -- copper is still copper; it's just stretched out.
4.) Chemical -- marshmallow reacted with heat and chemically changed.
What year did I graduate from Fonda?
2020
This term defines the study of matter and its properties.
Chemistry