The Significant figure/s of 98 g
"What is" 2 significant figures
The 3 Density equations
What is Density =Mass/Volume ,
Volume =Mass*Density ,
Mass=Volume/ Density
The number of rows and columns in the periodic table
What is 7 rows and 18 columns
The average Atomic Mass of Helium 
What is 4.0026 lbs
This person discovered Atoms have electrons
Who is JJ Thomson.
The Significant figure/s of 7.00 x 105
What is 3 significant figures
An object will float in water if it is above this number
What is 1 g/ml
The Alkaline earth metals are in this specific group
What is group 2
The Atomic # of Molybdenum

What is 42
This person discovered Atoms have Nucleuses
Who is Ernest Rutherford
The significant figure/s of 0.00907
What is 3 significant figures
This factor of density increases the temperature
What is Volume
This category of elements are in group 18 in the periodic table
What is Noble gases
The amount of Protons in Lead

What is 82
This person said atoms were made out of little particles.
Who is John Dalton
Round .0090825 to 3 significant figures
What is .00908
A 9 gram object gets placed in a bowl of water and raises the water level from 35.00 mL to 41.8 mL. This is the volume of the object
What is 6.8 mL
This table is split up by these groups
What Is Metalloids , Nonmetals, and Metals
This equation is how you find mass #
Protons + Neutrons =Mass #
This famous scientist created this theory 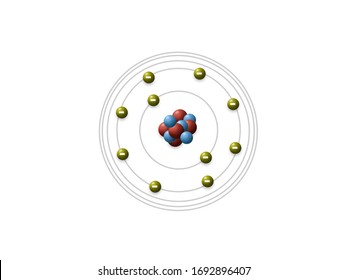
Who is Neils Bohr
Round .0903080209588004 to 10 significant figures
What is .09030802096
A 78 gram object gets placed in a cup of water and raises the water level from 198.35 mL to 223.5mL. This is the volume of the object
What is 25.15 g/mL
This group is the most reactive nonmetals and reacts with Alkali metals.
What is halogens,group 17
There are 94 protons and 94 electrons. The mass number is
What is 188
This person made the theory of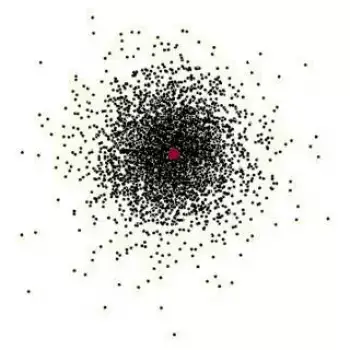
Who is Erwin Schrodinger