The code number of James Bond
007
Covalent bonds form between these types of elements
Name the compound made from chlorine and sodium.
Sodium Chloride
This particle defines the type of element.
The number of protons
the bonding of metal and non-metal
What is ionic bond
How does James Bond take his martini?
Shaken; not stirred
Atoms that have formed a covalent bond are said to do this with their electrons.
Share electrons
The balanced formula to describe a compound made between potassium and nitrogen. (ie. H2O is "H" two Oh.)
K3N
Bond between two oxygen atoms
covalent (double bond)
The name of the scientist that provides James Bond with gadgets
Q
The number of electrons that participate in a double bond
4
Name of this compound:
(NH4)2O
Ammonium oxide.
8
Type of bond formed between two nonmetals with different electronegativities
Polar covalent
Name of one of the actors to play James Bond.
Roger Moore, George Lazenby, Sean Connery; Timothy Dalton, Pierce Bronsnan
draw a Lewis Dot diagram showing a molecule of N2
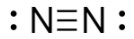
The formula of the compound made between magnesium and sulfate.
MgSO4
Columbic attraction (or positive to negative)
Type of bonding where electrons are transferred from one atom to another
Ionic Bond
What two actors have played James Bond the most?
Sean Connery and Roger Moore
4
Total numbers of protons and electrons in the polyatomic ion NH4+
Protons=11
Electrons= 10
Number of valence electrons (ion charge also acceptable)
Type of bond(s) in the compound: LiSO4
ionic and covalent