Alkene
If something has a pH of 12 is it more acidic or basic?
Basic
What is the most electronegative element?
Flourine
If you were to increase the temperature in a balloon, how would the pressure react in the same system?
The pressure would increase.
What type of bonding does C3H8 have?
London Dispersion Forces
What is the IUPAC name of this molecule?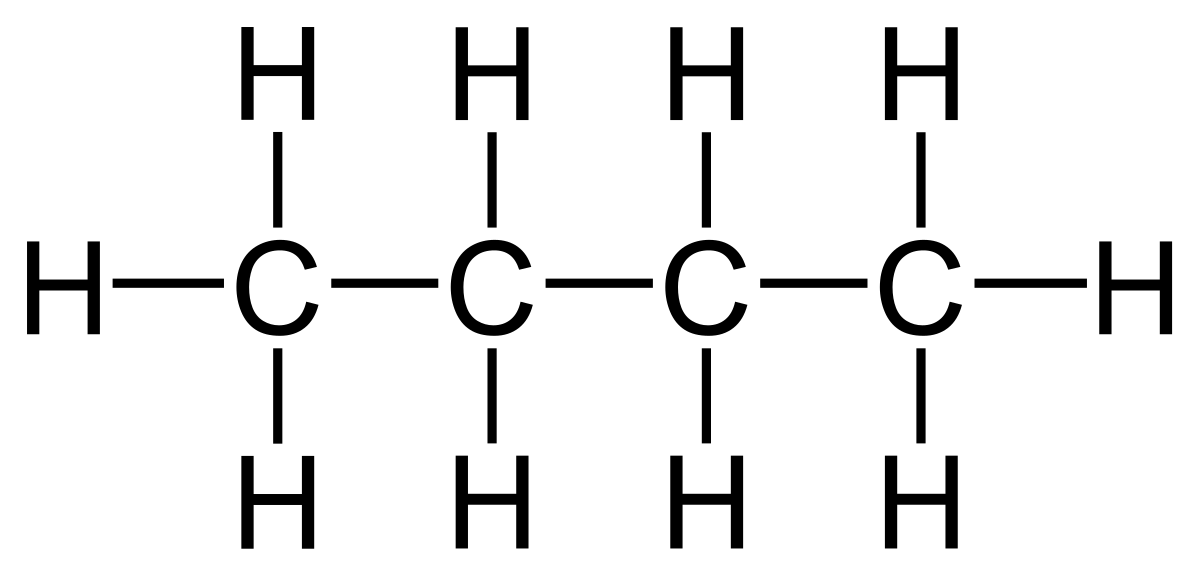
Butane
If you were to mix a strong acid and a strong base, roughly at what pH would it reach equivalence?
7
What is the trend for atomic size?
It increases down and across the periodic table.
If you increased the volume of a system then how would the pressure react?
The pressure would decrease.
What type of bonding do only polar molecules have?
Dipole Dipole Forces
What functional group does C2H6O have?
Hydroxyl Group
What pH is true neutral?
7
Which atom is the largest?
F
O-1
N-2
N-2
What is the gas law that shows a relation between volume and temperature?
Charles Law
Order the following bonding forces in order of weakest to strongest...
Hydrogen Bonding
London Dispersion Forces
Dipole Dipole Forces
London Dispersion Forces, Dipole Dipole Forces, Hydrogen Bonding.
What is the IUPAC name for this molecule?
Ethyl Ester (Acetic Acid)
What color does Phenolphthalein turn in basic solutions?
Pink
What molecular geometry does this molecule have?
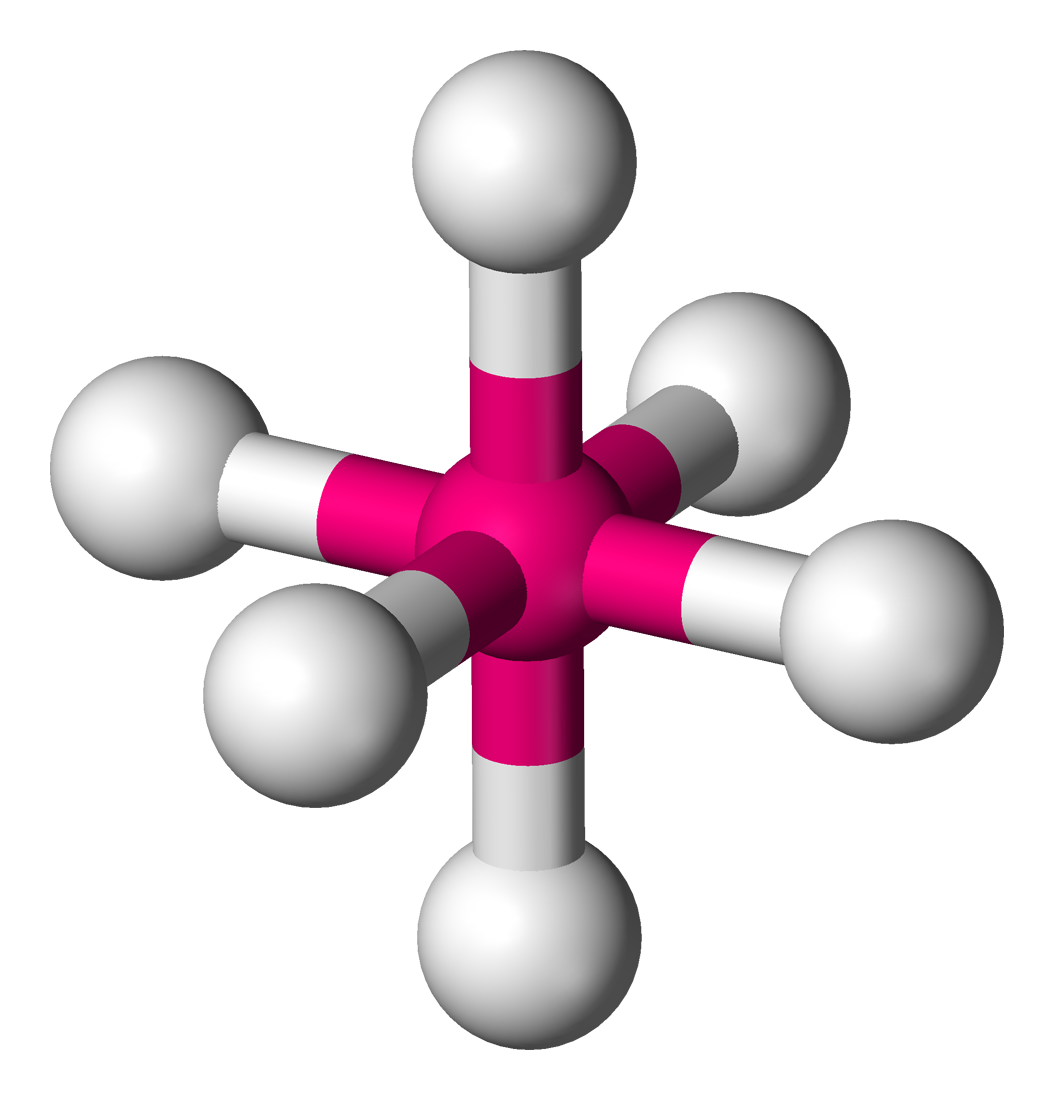
Octahedral
What is the ideal gas law?
PV = nRT
The ideal gas law, also called the general gas equation, is the equation of state of a hypothetical ideal gas.
Which molecule has the strongest inter-molecular forces?
C2H5O
C2H6O
C2H6
C2H6O
In chemistry, an enantiomer – also called optical isomer, antipode, or optical antipode – is one of two stereoisomers that are mirror images of each other that are non-superposable.
Do bases accept or produce hydronium ions?
How many sigma and pi bonds do 3 triple bonds have?
3 sigma
6 pi
If you increase the temperature in a system by a factor of 2 while also decreasing the volume by a factor of 2, then what is the overall effect on the pressure of the system?
Pressure will be increased by a factor of 4.
If Iron and Sulfur were to join together in a bond, what type of bonding would they exhibit?
Ionic Bonding