How many significant figures are in the numerical value: 0.0306 grams.
3 significant figures
This element has an atomic number of 30.
Zinc (Zn)
How many sulfur dioxide molecules are there in 1.80 g of sulfur dioxide (SO2)?
1.69 x 1022 molecules
What is the name of this gas law: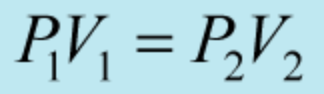
Boyle's Law
___ Na + ___ O2 --> ___ Na2O
4 Na + 1 O2 --> 2 Na2O
Which type of energy is associated with the position of the object?
potential energy
This shape belongs to which electron orbital?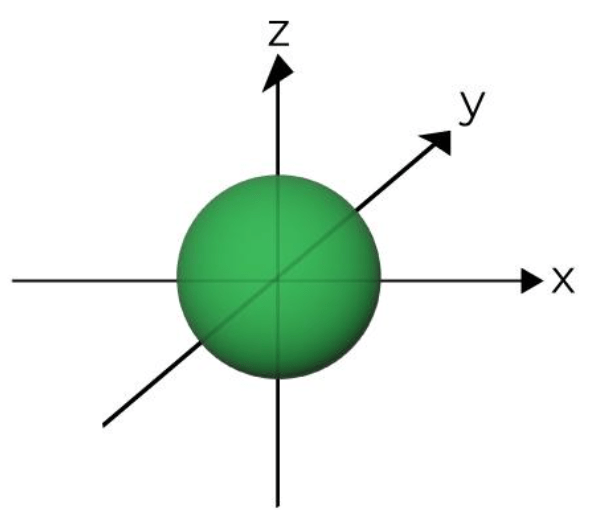
s orbital
N2O4
dinitrogen tetroxide
The SI unit used to measure mass.
kilograms (kg)
When forming an ionic compound, calcium loses electrons and has a positive 2 charge. Is Ca2+ a cation or an anion?
Cation
How many grams of CH4 are present in 6.890 moles of CH4?
110.6 g
What is the volume of 2 moles of nitrogen gas at STP?
44.8 L
___ Cs + ___ N2 --> ___ Cs3N
6 Cs + 1 N2 --> 2 Cs3N
Is the change in enthalpy positive or negative for an endothermic reaction?
positive (+)
Green light has a frequency of 6.01 x 1014 Hz. What is the wavelength (in nm)?
499 nm
H2SO4
sulfuric acid
Perform the indicated operations to the correct number of significant figures: (9.701 x 0.45) + 782.89
787.3
How many protons, neutrons, and electrons does an average vanadium (V) atom contain?
protons = 23
electrons = 23
neutrons = 28
What is the concentration of sodium ions in a 0.50 M solution of sodium phosphate (Na3PO4)?
1.5 M
If a gas at 37.0 °C is pressurized from 162 atm to 298 atm, what would be the final temperature in kelvin?
570 K
___ Na + ___ NaNO3 --> ___ Na2O + ___ N2
10 Na + 2 NaNO3 --> 6 Na2O + 1 N2
What is the specific heat capacity of aluminum if 210 joules of heat are needed to raise the temperature of 10.0 g of aluminum from 22 °C to 55 °C?
0.90 J/g*°C
Calculate the energy of a photon of light with a frequency of 8.5 x 1014 s-1
5.63 × 10-19 J
The chemical formula for the polyatomic ion, bicarbonate.
HCO3-
Convert 4.78 x 107 nanometers (nm) into centimeters (cm). 
4.78 cm
How many protons, neutrons, and electrons does the ion: Br- have?
protons = 35
neutrons = 45
electrons = 36
What volume (in mL) of C6H6 contains 8.50 x 1024 carbon atoms?
Density of C6H6 = 0.877 g/mL
210 mL
If 3.20 moles of a gas are at a temperature of 314 K and a volume of 83.7 L, what is the pressure of the gas?
0.985 atm
___ N2 + ___ O2 --> ___ N2O5
2 N2 + 5 O2 --> 2 N2O5
If 60 J of work is done by a gas, and the gas loses 150 J of heat to its surroundings due to the work, what is the change in internal energy?
- 210 J
Calculate the energy (in J) of a photon of radiation with a wavelength of 6.4 x 10-7 m.
3.1 × 10-19 J
Be3N2
beryllium nitride
 Convert 4.56 pounds to kg.
Convert 4.56 pounds to kg.
2.07 kg.
An isotope of this element contains 51 neutrons and 46 electrons.
Palladium (Pd)
Calculate the molarity for the following solution:
952 grams of ammonium carbonate are dissolved to make 1750 mL of solution
5.67 M
A balloon has a volume of 45.6 L at 298 K. What is the temperature of the balloon if the volume is decreased to 25.8 L?
169 K
___ Rb + ___ RbNO3 --> ___ Rb2O + ___ N2
10 Rb + 2 RbNO3 --> 6 Rb2O + 1 N2
A block of copper of unknown mass has an initial temperature of 65.4 ℃. The copper is immersed in a beaker containing 95.7 g of water at 22.7 ℃. When the two substances reach thermal equilibrium, the final temperature is 24.2 ℃. What is the mass of the copper block? The specific heat of water is 4.186 J/g ℃ and copper is 0.385 J/g ℃.
37.8 g
A nitrogen gas laser pulse with a wavelength of 337 nm contains 3.83 mJ of energy. How many photons does it contain?
6.49 x 1015 photons
Chemical formula for aluminum sulfide.
Al2S3